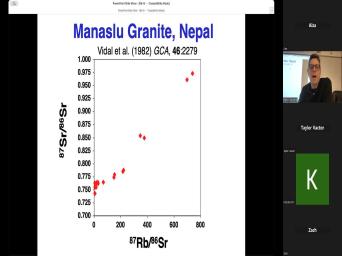
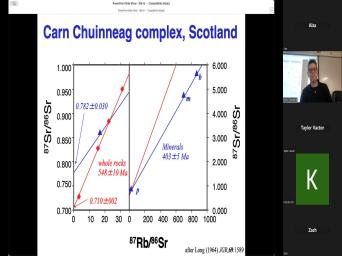
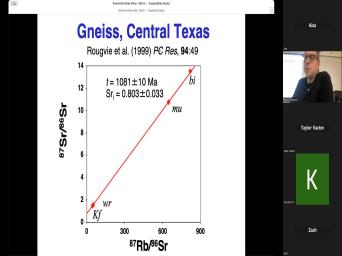
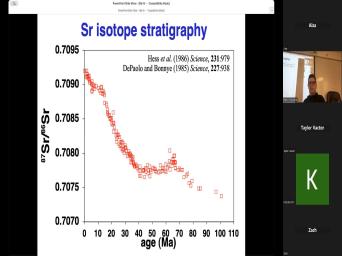
| 00:00 | Basic theory of very good. Uh basic theory of, of uh |
|
| 00:09 | And then some, some secondary theory the importance of this thing called a |
|
| 00:15 | temperature, which I'll get into in and in the middle of it |
|
| 00:19 | But the main thing to say about temperature, we're gonna talk about this |
|
| 00:23 | lot. So it's important that you a sense of what we're talking about |
|
| 00:26 | that because when we, when, you, when you date something, |
|
| 00:30 | you say this rock is a million old or 100 million years old, |
|
| 00:35 | you're doing is really telling what you , which when you date it, |
|
| 00:39 | matter what system you're dating, you're determining the last time that system say |
|
| 00:46 | in a Fels bar or lead in Zircon, whatever your system is you're |
|
| 00:51 | about, when were the last time system was at its closure temperature, |
|
| 00:56 | temperature at which the system becomes closed the clock starts ticking. It's important |
|
| 01:02 | recognize that sometimes the closure temperature can really, really low as low as |
|
| 01:08 | 70 °C, which means that if rock is buried to say you got |
|
| 01:14 | sandstone, you bury that sandstone to kilometers. It's gonna start resetting some |
|
| 01:23 | these isotopic clocks uh which can be useful for learning about how deep this |
|
| 01:28 | was buried. Uh On the other , if you're not interested in how |
|
| 01:32 | it was buried, but when it deposited, you're gonna need a different |
|
| 01:35 | . You're gonna need one that's insensitive being buried down to 100 degrees. |
|
| 01:40 | So we'll start now with some, basics of radioactivity, we'll move into |
|
| 01:47 | some description of closure temperature and from , we'll move in and start talking |
|
| 01:54 | individual isotopic systems and their best geologic when we do and don't want to |
|
| 02:01 | them next week. No, I'm the end of this. Yeah, |
|
| 02:06 | did. You did don's part first you'll have a test not next |
|
| 02:10 | but the week after. Right. . OK. So today and tomorrow |
|
| 02:15 | gonna be going through the techniques depending how we do, depending on |
|
| 02:18 | what kind of interactions we have. will uh probably have next Friday. |
|
| 02:24 | be largely um discussing sort of case and examples and getting you guys to |
|
| 02:30 | of interact. And I'll give you now that we've done this, which |
|
| 02:33 | the best way to go. Um that'll be really a practice for the |
|
| 02:38 | . I will ask you questions about this situation. Uh What system is |
|
| 02:43 | appropriate for the answering this geologic OK. So we can start with |
|
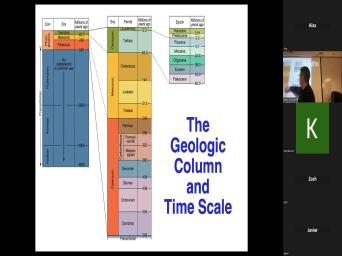
| 02:52 | , right? We know that the age of rocks starts with the principle |
|
| 02:56 | superposition. That's nice. Um We even be able to go up to |
|
| 03:01 | rock like this and figure out that got the trilobites in it, which |
|
| 03:05 | it's paleozoic or dinosaurs in it, means it's mesozoic. Um But, |
|
| 03:11 | that was done, you know, time ago. But after a |
|
| 03:14 | somebody figured out that, you when the dinosaurs went extinct, |
|
| 03:18 | when did the dinosaurs go extinct? me later, after that, the |
|
| 03:28 | of the Cretaceous. But how many ago was that? 65? |
|
| 03:33 | it's now, now folks think is 66 how do we figure, where |
|
| 03:38 | that? 66 number come from? we had dinosaurs in this rock and |
|
| 03:45 | dinosaurs here. Somebody wants to say is 66 million years. How do |
|
| 03:48 | figure that out? Where does, does this notion? I mean, |
|
| 03:52 | got, you know, that you've the geologic column here. It |
|
| 03:56 | right? The geologic column has all names on here. What, how |
|
| 04:02 | we define Jurassic rocks? What makes rock? A Jurassic rock? |
|
| 04:16 | that actually can, I mean, me. Well, no, I |
|
| 04:22 | , a rock is the, I , the original definition of Jurassic rocks |
|
| 04:25 | rocks that have Jurassic fossils and that may sound circular, but it |
|
| 04:31 | isn't we've, we've de, we've that these are the fossils that are |
|
| 04:35 | and Don talked with you LA for last times about different bio stratigraphic uh |
|
| 04:41 | of various fossils. This one's useful this and this one's useful for |
|
| 04:44 | So, Jurassic fossils, Jurassic rocks the rocks that have Jurassic fossils in |
|
| 04:49 | . And we pretty much agreed what Jurassic fossils are and that ain't gonna |
|
| 04:54 | . That's a Jurassic fossil that But, but these numbers are actually |
|
| 04:59 | to change and we'll talk about let's, uh, I don't |
|
| 05:03 | we'll get to that in a Well, actually, we can go |
|
| 05:06 | so we can, we can you know, I said Jurassic, |
|
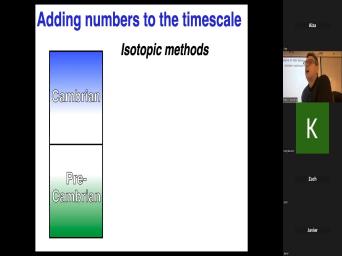
| 05:11 | do, let's do Cambrian and pre again, Cambrian rocks are rocks with |
|
| 05:14 | fossils in it and that's gonna be . We're not gonna, we're not |
|
| 05:18 | later on say that's not, that's Cambrian. No, we're never gonna |
|
| 05:21 | that because Cambrian fossils have been agreed . They are, they look |
|
| 05:25 | There's something that we're gonna draw a there, that's not the same |
|
| 05:29 | Um So that's a paleontological definition. not gonna mess with that, but |
|
| 05:36 | want to know and that's gonna be same, whatever, whatever Strat interval |
|
| 05:40 | choose the legacy needs to also paleontological determined and that's not gonna change. |
|
| 05:47 | But how many years ago is That's a concern, right? Because |
|
| 05:53 | , I mean, it's, it's that fossils help us this way. |
|
| 05:56 | if we're gonna answer some geologic we have to know how fast things |
|
| 05:59 | happen. Um A mentor of mine I learned a lot about geo chronology |
|
| 06:04 | was, was famous for saying, dates, there are no rates you |
|
| 06:08 | to figure out what rate of geologic you're talking about. You got how |
|
| 06:12 | years are involved? The fact that in this particular Ammonite zone doesn't really |
|
| 06:17 | you that it tells you that it's below the next Amoy zone. But |
|
| 06:20 | we know how many years ago, one of those were, we're not |
|
| 06:23 | about rates will not be able to , well, you know, anthropogenic |
|
| 06:27 | change is faster than any time in , in the past. Well, |
|
| 06:30 | you have numbers, you can't say like that. When did, how |
|
| 06:34 | did something, how, how fast this extinction event happen? Well, |
|
| 06:38 | this Ammonite zone and that Amy zone tell you at the time. So |
|
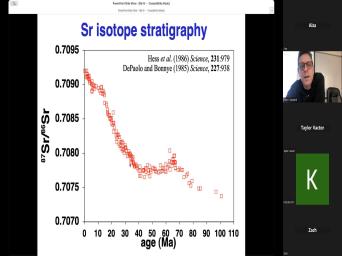
| 06:42 | are we gonna figure out the Well, this, this just shows |
|
| 06:46 | that the, the nature of of these time of these, of |
|
| 06:50 | answers has changed over time. here's just the last, the ESC |
|
| 06:56 | are, you know, we've, shown ECL my, just the last |
|
| 07:01 | million years on this diver and what have along the, the, |
|
| 07:05 | the way here is different publications and about when these uh periods were |
|
| 07:13 | And remember these numbers haven't changed because changes in our understanding of the |
|
| 07:18 | But back in 1937 somebody said that boundary between the EFC and the legacy |
|
| 07:24 | down here at 50 48 million Well, in 1961 somebody said what |
|
| 07:29 | is more like 36 and then it around until now it's at about 3033 |
|
| 07:36 | something. All of these have moved . Um Some, a lot more |
|
| 07:40 | that when I was an undergraduate many years ago, I was told |
|
| 07:45 | the precambrian Cambrian boundary was 570 million ago. And that was the best |
|
| 07:50 | could do at the time. now we have a pretty good idea |
|
| 07:52 | it's about 542 plus divis one. why do these things change? Two |
|
| 07:59 | happen? Is that well, we're gonna, these numbers, all |
|
| 08:03 | these numbers, all of, you , where, where we put these |
|
| 08:07 | is based on some sort of isotopic of ages. Um And so we're |
|
| 08:13 | do that and, and let's uh , so let's go through some |
|
| 08:19 | How help me help me out what are ways in which we could |
|
| 08:24 | isotopic methods to these sedimentary rocks to their age and, and be give |
|
| 08:33 | as much latitude as possible. Imagine outcrop, imagine a technique, imagine |
|
| 08:37 | rock. What would be helpful to the age of this thing beyond |
|
| 08:42 | it's got Cambrian fossils in it. . The co, the content, |
|
| 08:56 | fossil fossil that, that fossils don't us how many years ago it |
|
| 09:01 | Fossils will tell us that it's we already know that. Go to |
|
| 09:06 | boundary and use an isotopic measurement to what the age of the boundaries are |
|
| 09:12 | boundary. Um, that's, you're on the right track there. |
|
| 09:16 | come I can't see? Oh, don't have your thing on? Uh |
|
| 09:20 | . Yeah, there you are. . Hello Taylor. Um You're absolutely |
|
| 09:25 | . So, but, but but saying going to the boundary is |
|
| 09:28 | little bit complicated because these are sedimentary . It's difficult to date a sedimentary |
|
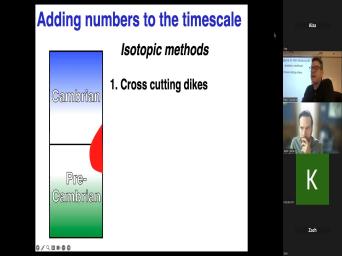
| 09:33 | . Let me just let me just ahead here and say, here's one |
|
| 09:36 | we could use by going to the . As Taylor suggests, we could |
|
| 09:40 | a cross cutting relationship if we had rock that cross cut this, this |
|
| 09:46 | paling to logical boundary. And I say it's an IOUs, an igneous |
|
| 09:51 | . We can date that igneous rock some precision and suppose we dated that |
|
| 09:56 | . What would it tell us about , uh about the age of the |
|
| 10:02 | significant transition? We've got an age that red rock there. What does |
|
| 10:07 | tell us about what we're really interested ? Yeah, the, the, |
|
| 10:14 | paleontological interesting transition has to be older whatever we dated in this exam. |
|
| 10:21 | that's great. It's older than now it came, if it came to |
|
| 10:24 | that we were looking at the Cambrian boundary and we looked at a dike |
|
| 10:28 | they gave us 50 million years. , then we're stuck with saying the |
|
| 10:32 | is older than 50 million. Uh , you know, maybe somebody else |
|
| 10:37 | gonna find another one and another one another one and you get better. |
|
| 10:41 | That's a good way. But, as I say, you know, |
|
| 10:43 | might have a dike that's way much and doesn't tell you all that |
|
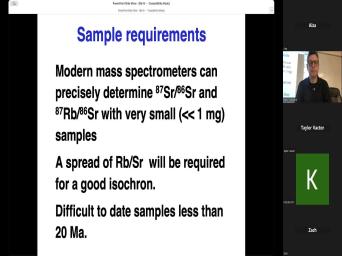
| 10:47 | Um What about dating individual minerals say, a sandstone, we could |
|
| 10:54 | a bunch of these minerals. What that tell us? And this is |
|
| 11:00 | we'll talk a lot about tomorrow is dating of detrital minerals and how |
|
| 11:04 | tells us something about provenance, but tells us about, about uh uh |
|
| 11:09 | histories of basins. It's a very deal and, and you can also |
|
| 11:12 | us, you can also use this to tell us about the age of |
|
| 11:15 | sediment, but it has a, has a similar limitation as the one |
|
| 11:19 | just described. What's the limitation here I date some of these grains? |
|
| 11:24 | , what does that tell us about age of the sedimentary rock in which |
|
| 11:27 | are obtained? Yes. Mhm. , yeah, of course, of |
|
| 11:42 | . But just, just as we only know the context in this |
|
| 11:46 | But what, what did you say time? We, we, we |
|
| 11:50 | us again what the, what this us? Thank you. The dike |
|
| 11:55 | younger. So the, the, , the Cambrian rocks have to be |
|
| 11:58 | than the, the rock we What about in this case? |
|
| 12:05 | you can't deposit something until it So we date these grains and we |
|
| 12:11 | that the sedimentary deposit must be younger that. If we were to date |
|
| 12:16 | whole bunch of them, say 100 500 grains out of a sandstone. |
|
| 12:21 | that, that can be done relatively these days. With the technology we |
|
| 12:25 | popping off a couple 100 grains is a big deal. Let's say we |
|
| 12:29 | 200 grains in this context to determine age of this sediment, which one |
|
| 12:35 | those 200 grains is gonna be the important to us. The youngest. |
|
| 12:47 | . The, the youngest one, all those older ones, we're gonna |
|
| 12:52 | repeat the same information we learned. we look at 200 grains and we |
|
| 12:57 | attention to the youngest one. The must be younger than that still. |
|
| 13:01 | right. So that's so, so we had a, a detrital date |
|
| 13:05 | then a cross cutting dike. We're in, we're narrowing in, we |
|
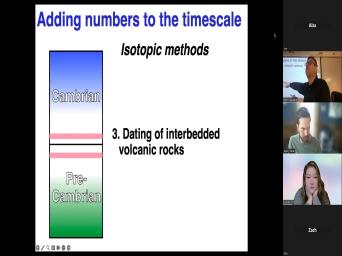
| 13:08 | do both at the same time, ? Um The third way, the |
|
| 13:13 | way is to do this is to lucky and find some volcanic rocks that |
|
| 13:19 | like that. And I, I to say that the best way to |
|
| 13:24 | a sedimentary rock is to date an rock that's easier said than done. |
|
| 13:30 | course, because you have to find outcrop of this sort where you have |
|
| 13:34 | , a volcanic rock above and The thing you're interested in if you |
|
| 13:39 | find. And, and the reason , is this is so much, |
|
| 13:42 | best way is because the, the , the dating and the interpretation of |
|
| 13:48 | date you get from a volcanic rock usually very straightforward. If you're trying |
|
| 13:53 | date, if you're trying to, date other rocks that are plutonic rocks |
|
| 13:56 | may have slow cooling involved, that things. And we'll talk a lot |
|
| 14:00 | why the rate of cooling will complicate issue depending on which system we |
|
| 14:05 | But no matter what isotopic system we're , um a volcanic rock should basically |
|
| 14:11 | the same answer doesn't really matter because rocks go from very hot to very |
|
| 14:15 | , very fast. We understand the this volcanic rock was erupted in a |
|
| 14:22 | or in a week or something like , right? Whereas sedimentary rocks could |
|
| 14:25 | thousands of years to accumulate a similarly uh deposit. So we can interpret |
|
| 14:31 | , we understand what it is. so that's the best way an even |
|
| 14:35 | way would be this, right, you find our, our interbedded volcanic |
|
| 14:40 | are right smack at top and And this is why we know that |
|
| 14:44 | Cambrian precambrian boundary is 542 plus or one because they really found one of |
|
| 14:49 | places in Namibia. There's the precambrian boundary. There's a rite under |
|
| 14:54 | There's a rite up here. yeah, we got it. That's |
|
| 14:58 | best way because ry lights can be , they can be dated unambiguously. |
|
| 15:03 | what we wanna do. That's my . That's why the boundaries keep |
|
| 15:08 | Well, there's two reasons these boundaries changing is that people keep finding better |
|
| 15:13 | . And there's also is that, know, this, this, |
|
| 15:16 | this, this history goes back to when the technology wasn't as good. |
|
| 15:21 | know, when you couldn't carry a in your pocket that also made c |
|
| 15:26 | telephone calls. Um The advancement of machines that make these measurements has allowed |
|
| 15:32 | to uh to look at different kinds samples, smaller samples, greater |
|
| 15:37 | all the, all the machines are so that this is involved. This |
|
| 15:40 | a measurement. Um And so the gets better all the time every 10 |
|
| 15:45 | , somebody comes out with an even mass spectrometer that's gonna do even better |
|
| 15:49 | smaller samples and finer and older and . Um But none of that's any |
|
| 15:54 | unless you have one of these. ? And so over the years |
|
| 15:58 | and this is, this is kind tricky too because imagine what do you |
|
| 16:02 | to find this outcrop? You need understand that this is the palely interesting |
|
| 16:06 | and you further need to recognize that a real life that we can do |
|
| 16:11 | sometime and, and this place in that I'm familiar with was actually the |
|
| 16:16 | thought they had some, some volcanic . So they got the geologist to |
|
| 16:20 | into the field with them and find rock. You know, they, |
|
| 16:22 | have 11 group of people who understands fossils, one group of people who's |
|
| 16:26 | so much with the fossils, but the other stuff. So that eventually |
|
| 16:32 | . And so that's how we can numbers to the Strat democratic column, |
|
| 16:36 | was originally based on fossils. We're gonna change uh when the Jurassic |
|
| 16:41 | but all the time we might be the numbers. Although it's gonna probably |
|
| 16:45 | , if we're doing it well, , the, the, the changes |
|
| 16:49 | , as we progress are gonna get and smaller, we're gonna eventually start |
|
| 16:53 | that that's the time I remember it have been 1010 or 12 years |
|
| 16:57 | Now I went to a GS A and there was a symposium that was |
|
| 17:02 | about the, um, all about , the age of the, where |
|
| 17:08 | it? It was the age of , yeah, the Jurassic Triassic boundary |
|
| 17:14 | they were, you know, they fiddling around whether it was 2, |
|
| 17:17 | or 2, 13 and that sort thing. Um, and so now |
|
| 17:22 | , it's some number that's getting smaller smaller used to be. It was |
|
| 17:25 | 210 plus or minus 20 now it's to 212 plus or minus a |
|
| 17:30 | I should say that in general um, when we date rocks, |
|
| 17:37 | is something you should keep in mind we're, as we're thinking about |
|
| 17:41 | um, for simple dating for like a rite. Um The, the |
|
| 17:48 | is such that we ought to be to date that rock to within half |
|
| 17:52 | percent. The uncertainty should be half percent or better. That means if |
|
| 17:56 | 100 million years old should be 100 or minus 0.5 that's entirely normal. |
|
| 18:02 | special anymore. Used to be, know, 40 years ago. If |
|
| 18:06 | told somebody you dated something to plus minus a half, they would, |
|
| 18:09 | know. Oh, yeah, you do that. But nowadays, if |
|
| 18:12 | , you know, if you say plus or minus one, you |
|
| 18:15 | the geologists in the crowd are gonna why so bad? What went |
|
| 18:21 | Uh That's how good things are Half a million, half a percent |
|
| 18:25 | , is not special at all. some cases, it can get down |
|
| 18:28 | 1/10 of a percent. Uh And , you know, and that's part |
|
| 18:32 | the, the, the, the improvement of the technology as this |
|
| 18:35 | better and better as we can The difference between signal and noise better |
|
| 18:40 | our machines. We can start resolving and smaller things. And so now |
|
| 18:44 | can start talking about, you what was, what was the rate |
|
| 18:47 | evolution of something that only took six 7 million years? If you |
|
| 18:51 | if you can't, if you uh, if you can't, |
|
| 18:55 | date something to within plus or minus , you're never gonna resolve something that |
|
| 18:59 | took a million years. But if can resolve it to plus or minus |
|
| 19:03 | you can start talking about million year that occurred 300 million years ago. |
|
| 19:11 | All right. So of course, of this is based on the regular |
|
| 19:14 | decay of some chemical elements. And we'll mention, as I've, as |
|
| 19:19 | hinted to, and as we'll mention geo chronology, as we'll discuss quite |
|
| 19:24 | bit in these lectures is also capable providing information about the thermal history of |
|
| 19:30 | samples, not just when they were , but what was their thermal |
|
| 19:34 | When were they at certain temperatures? that's because as I mentioned, many |
|
| 19:38 | these systems are sensitive to heating you heat them up a little bit |
|
| 19:43 | the, the clock that we are will be reset, we start over |
|
| 19:47 | that's a function of temperature. And good news is is that we have |
|
| 19:51 | different clocks that have many different So we can understand whether or not |
|
| 19:56 | rock was heated to only 70 degrees maybe it was 100 and 50 |
|
| 20:00 | maybe it was 400 degrees, we tell the difference by looking at these |
|
| 20:04 | systems. Um And so this can be applied to all sorts of |
|
| 20:10 | These are the two things that, I've applied it mostly to. |
|
| 20:15 | is, you can understand, the age and uplift of, of |
|
| 20:19 | of mountain belts because as rocks are uplifted, they're being cooled off. |
|
| 20:23 | so as they hit certain certain you can sort that out. Um |
|
| 20:28 | you can understand the history of basins well or just the Strat democratic uh |
|
| 20:33 | of basins. So we'll return to soon. But I'm gonna talk a |
|
| 20:38 | bit of physics now to remind us of some stuff that we probably already |
|
| 20:42 | . Um when we're gonna talk about radioactivity, we gotta remember that uh |
|
| 20:47 | are made up of electrons, protons neutrons. Um We talk about the |
|
| 20:53 | characteristics of an, of an atom largely determined by the number of |
|
| 21:00 | Carbon. Is that thing with six . Uranium is that thing with 92 |
|
| 21:06 | , but they have neutrons coming along the ride. Um And so we |
|
| 21:11 | talk about the total number of neutrons protons as the atomic weight. And |
|
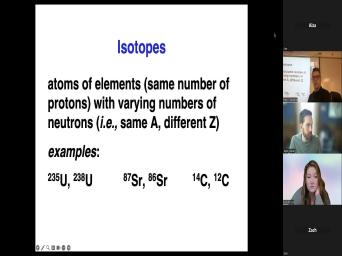
| 21:15 | what it means when we talk about 12 or carbon 14 or uranium |
|
| 21:20 | That's the total. And it's important , because we keep track of the |
|
| 21:27 | because some isotopes are radioactive and some are not radioactive even in the same |
|
| 21:32 | . For example, carbon 14 and 12, carbon 12 is six protons |
|
| 21:37 | six neutrons. Not radioactive. Carbon is six protons, eight neutrons. |
|
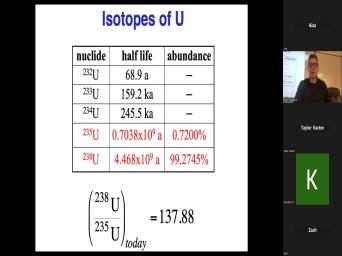
| 21:42 | is radioactive. Um Other examples on slide include um uranium 238 and uranium |
|
| 21:50 | . Both radioactive, different different rates decay, but they're both radioactive |
|
| 21:56 | We'll talk a little bit about that a little in a little while two |
|
| 22:00 | of strontium. There are four isotopes strontium that we'll talk about. 42 |
|
| 22:04 | them are just mentioned here. All of the isotopes of strontium are |
|
| 22:09 | They're not, they're not changing just carbon 12 is not changed. |
|
| 22:13 | one difference is is that strontium 87 the daughter product. It is the |
|
| 22:19 | by the decay of rubidium 87. , whereas carbon eight or carbon 12 |
|
| 22:24 | Stron 86 are not changing the amount strontium 87 in the world or in |
|
| 22:30 | sample is increasing if you have some in that sample. So we say |
|
| 22:35 | say that such an element, we that an element like uranium 2388 is |
|
| 22:40 | . An element like Stron 87 is , it is produced by the decay |
|
| 22:44 | something else. So I use some these terms already, we talk about |
|
| 22:50 | radioactive elements. We say they are parents and they decay to something we |
|
| 22:55 | the daughters. The daughters may themselves unstable. Sometimes you decay to something |
|
| 23:01 | is unstable, which decays to something decays to something and eventually that decays |
|
| 23:06 | something that is stable. Um, the case of uranium and uh and |
|
| 23:11 | heavier than lead, uh, we to decay down to lead, lead |
|
| 23:15 | the last thing on the periodic table always stable, everything higher than that |
|
| 23:20 | gonna be radioactive. And so all uranium atoms, all they uranium and |
|
| 23:25 | , which we use as chronometers, decay down to lead, but over |
|
| 23:31 | or seven or eight steps. the key thing is that the rate |
|
| 23:36 | which this decay occurs is constant and . And this is actually a bit |
|
| 23:41 | a strictly speaking, I shouldn't say rate is constant, but I'll get |
|
| 23:45 | that in a minute. But if know how it decays and we can |
|
| 23:49 | the amount P present of the parent the daughter, then we can calculate |
|
| 23:54 | age. That's all there is to . So I'll go into the mathematics |
|
| 23:58 | that in a minute. Uh But that, let's just talk about the |
|
| 24:03 | that we have actually uh uh that happened, there are several kinds |
|
| 24:08 | decay and the type of decay does relate to the rate of decay. |
|
| 24:13 | can have some things that decay really by alpha decay and some things really |
|
| 24:17 | . So that's, that's not a . Um One way in which something |
|
| 24:23 | decay, there are two different kinds beta decay, one called beta minus |
|
| 24:27 | which we basically transform a neutron into proton and an electron. And so |
|
| 24:35 | changing one of the things in the to another thing in the nucleus, |
|
| 24:40 | total number doesn't change. So in case, we have potassium 40 it's |
|
| 24:45 | into calcium, 40. Kept the didn't change. But, but we |
|
| 24:50 | it calcium now because it has uh protons. And by the way, |
|
| 24:55 | me, I guess I didn't talk , I didn't talk about this nomenclature |
|
| 24:59 | shown here. This is how we write um isotopes fully. You write |
|
| 25:04 | K for potassium, right? And 40 up here tells us how many |
|
| 25:10 | neutrons and protons. This 19 down is actually superfluous because potassium is already |
|
| 25:17 | thing we have defined as the thing 19 protons. So you don't have |
|
| 25:21 | put that in there. But it's because you don't have to remember which |
|
| 25:24 | is. Potassium is at 19, at 17. Um It also helps |
|
| 25:28 | you're doing a diagram like this where can see look what happened. The |
|
| 25:31 | became 20 but the 40 say So what happened? We took a |
|
| 25:36 | turned it into a proton and now call it calcium. So it is |
|
| 25:40 | new element. It's chemically different now it's got more protons. It also |
|
| 25:45 | off some energy and an electron and other stuff. The energy here is |
|
| 25:50 | . We'll get to the Q means . Um uh It's just a silly |
|
| 25:55 | . It doesn't really help. Um decay or beta positive decay is the |
|
| 26:01 | . We transform a proton into a . And so here we've gone from |
|
| 26:05 | , 18 to oxygen, 18, is nine, oxygen is eight. |
|
| 26:10 | same concept uh that last one is geo geo geologically useful one. This |
|
| 26:16 | not so much. Um There's also uh a thing called electron capture and |
|
| 26:24 | can decrease um the to the, number of protons without changing the mass |
|
| 26:32 | taking an electron from one of the shells and creating a neutron from a |
|
| 26:39 | , you take an electron or they fuse together, they become a |
|
| 26:43 | . So that's a, that's a that happens uh in potassium argon, |
|
| 26:48 | talk about that later. And then , alpha decay. And this is |
|
| 26:53 | example where we do change the total of, of uh the uh |
|
| 26:57 | the, the, the atomic number the thing uranium 238 will decay to |
|
| 27:04 | 234. And then what's given off is what's called an alpha particle or |
|
| 27:09 | helium nucleus. Four pro it's got massive four, it's got a protons |
|
| 27:15 | two. So that means two protons two neutrons are tossed out. Um |
|
| 27:20 | here's where we actually lose mass. the other case, it all goes |
|
| 27:24 | inside the probe in the nucleus. here we're tossing things out. |
|
| 27:28 | it turns out, as I thorium 234 is itself unstable. It'll |
|
| 27:32 | to something else. But this is , the first decay on the |
|
| 27:36 | something else. When we say uranium decays, we sometimes skip this stuff |
|
| 27:41 | the middle and say uranium 238 decays lead 206 because usually because all the |
|
| 27:47 | in the middle here happens pretty We can kind of ignore it, |
|
| 27:51 | strictly speaking, there are, there steps along the way. Uh but |
|
| 27:56 | not gonna worry about all those steps it just to define ALPH, here's |
|
| 28:01 | example of alpha decay in which we have to worry about steps because it |
|
| 28:04 | finishes after one. So 147 decays Nemi 143 plus uh helium. So |
|
| 28:14 | are different ways in which we can . Um We're gonna discuss examples of |
|
| 28:19 | of these uh but how it decays not hugely important for what we're going |
|
| 28:23 | worry about. Um one other uh that's uh gonna be important um because |
|
| 28:32 | ha it happens in nature, but gonna have, we're gonna pay more |
|
| 28:35 | to when it happens artificially. And is something called the NP reaction. |
|
| 28:40 | NP reaction is when you take energetic and throw them into a nucleus and |
|
| 28:45 | knock out a proton NP means N in P goes out. And this |
|
| 28:50 | to this is how carbon 14 is . Carbon 14 is produced from the |
|
| 28:56 | in the air nitrogen's up here And, and a cosmic ray just |
|
| 29:00 | and hit one of those nitrogen atoms that, that cosmic ray had a |
|
| 29:04 | going fast enough, hit that nucleus out a proton. It's not nitrogen |
|
| 29:10 | . It's carbon now. And because carbon with eight protons and 686 protons |
|
| 29:16 | eight neutrons, it's unstable. It decay. Uh It actually decays right |
|
| 29:21 | to nitrogen, but that's another Um But that nt reaction is another |
|
| 29:26 | in which we can make something This will come into play because we |
|
| 29:31 | going to do this in the lab on to produce something argon 39 which |
|
| 29:37 | important for um improving the potassium argon system. But we'll, we'll probably |
|
| 29:43 | about that tomorrow morning. Um OK. One more way that that |
|
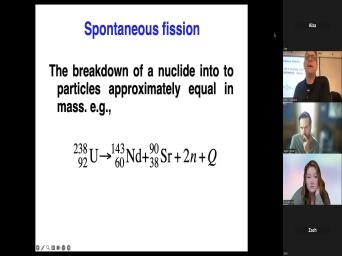
| 29:49 | can decay is by something called spontaneous in which a large nuclide like uranium |
|
| 29:58 | will on occasion not just toss off uh a helium atom but will actually |
|
| 30:04 | into two pieces that are about the size. Here. We've got an |
|
| 30:08 | here is uranium 238 decays to me give me 143 inch at night. |
|
| 30:14 | not always the same two things. this is just an example of things |
|
| 30:18 | can happen and this is important and we'll talk about this tomorrow and |
|
| 30:25 | is one of the most, one the thermal history methods that has the |
|
| 30:31 | uh history of application in the oil is fishing track dating. It's called |
|
| 30:36 | fishing track. When you make, , when this fishing in occurs, |
|
| 30:40 | got some uranium in your crystal and fishing occurs. And these two guys |
|
| 30:44 | really big, right? They toss , they, they're actually shot out |
|
| 30:47 | this place with some energy and they so big that they shoot through the |
|
| 30:51 | of the crystal and create a damage in that crystal. Why is that |
|
| 30:56 | ? Because if you heat that crystal a little bit that damage zone goes |
|
| 31:01 | and the heating, the temperature at that damaged gun goes away is about |
|
| 31:06 | °C, which if you're interested in oil business is an excellent temperature to |
|
| 31:10 | worried about. That's the temperature at we start making oil, right? |
|
| 31:14 | , um we'll talk more about that but fish and fish and track |
|
| 31:19 | You've ever heard of fishing trap Good? No, that's fine. |
|
| 31:23 | it's a big deal, especially for thermal histories of basins because it has |
|
| 31:28 | a low closure temperature. This damage in a crystal and you can look |
|
| 31:34 | it in a microscope. See that's , that thing there was produced when |
|
| 31:37 | fission broke down, when the fission and, and, and, and |
|
| 31:41 | this crystal and you can look at , you can measure it. It's |
|
| 31:43 | tiny little thing about 14 for about microns long, this this thing. |
|
| 31:48 | you're looking at it at a high but you can, you can find |
|
| 31:52 | . And uh what's very cool about is from the geological perspective is if |
|
| 31:57 | crystal say that was, say that was in a ry light, it's |
|
| 32:02 | forms and it starts occasionally efficient if were to erode that rhyolite on |
|
| 32:08 | Uh oh the, the, let just say the, the crystal |
|
| 32:11 | this is most commonly associated with is mineral appetite. Appetite has a lot |
|
| 32:16 | uranium and it's very susceptible to this . Appetite gets eroded from your uh |
|
| 32:21 | your rhyolite or your granite and gets into your sandstone. If that, |
|
| 32:27 | you're interested in knowing has that sandstone been buried deep enough to make |
|
| 32:33 | Well, one thing you can do look at those, look at those |
|
| 32:37 | in your sandstone and have they been ? You, you might pay, |
|
| 32:41 | might have a good idea of when sandstone was deposited. This is a |
|
| 32:45 | sandstone. If that is an oil , then some of those appetites are |
|
| 32:52 | have ages when we did them in lab, they're gonna be younger than |
|
| 32:56 | . They're in a Miocene rock. know that's a Miocene rock from |
|
| 32:59 | from the, from the uh But if that, if that's been |
|
| 33:04 | into the oil window, the appetites gonna start over, they're gonna have |
|
| 33:08 | younger than the deficit. Um, said that already. Ok. |
|
| 33:18 | oh, and this is another important . This is great news for |
|
| 33:21 | Geologists, radioactive decay is independent of or pressure. We don't have to |
|
| 33:26 | about things being uh uh decaying faster slower as they are buried down deep |
|
| 33:32 | the earth. That's not a That's great news because otherwise that would |
|
| 33:36 | a huge complication. Um Now, I said, we know the, |
|
| 33:41 | rate of decay is constant and that's a mistake. But because, because |
|
| 33:47 | in fact, impossible to predict when given nucleus will decay. If we |
|
| 33:52 | a uh that the uranium atom sitting on the table, there's nothing about |
|
| 33:58 | atom that says it sticks into However, we know that if we |
|
| 34:03 | to line up a million of we would, we'd have, we'd |
|
| 34:07 | that what percentage of them would decay the next time period, next |
|
| 34:12 | they all have this same probability. over time people behave like that, |
|
| 34:17 | it's a bit like it's a bit if we had, I used to |
|
| 34:20 | this experiment or demonstration in physical I do this demonstration when I'd have |
|
| 34:27 | big 100 person class. But you 100 person class and you also need |
|
| 34:31 | with coins in their pocket, which happen anymore. But if you |
|
| 34:34 | we all had, if we all coins in our pocket, we could |
|
| 34:38 | a coin. Right? And if in this room here, there's only |
|
| 34:42 | of us here. We flipped the . We'd expect to get four |
|
| 34:45 | right? What if we went um, what if we went to |
|
| 34:50 | Cougars basketball game on Saturday? And asked everybody to flip a coin. |
|
| 34:55 | many heads are we expecting to There was about 7000 people at the |
|
| 35:00 | game. So there'll be about 3500 . So there's, but there's |
|
| 35:05 | there's that the, the fact that got so many more heads in that |
|
| 35:08 | example, doesn't say anything about The quarters are different just that there |
|
| 35:12 | more of them. But so in the example of flipping a |
|
| 35:18 | we know that the probability is one . In the case of a, |
|
| 35:22 | uranium item, the probability is one of 10 billion in the next |
|
| 35:27 | OK. So that's what doesn't Um And so we can, we |
|
| 35:32 | , we can uh describe that mathematically the probability of decay in some small |
|
| 35:37 | interval. DT is gonna be DT where LAMBDA is this uh proportionality |
|
| 35:44 | for different things, you know, use a different lambda for, |
|
| 35:48 | for different, for uranium or potassium whatever. Um, and so the |
|
| 35:54 | at which these things happen, whether the eight of us here flipping the |
|
| 35:58 | or 7000 people in, in the stadium or maybe we wait a few |
|
| 36:03 | , we go to an Astros There'll be 40,000 people, you flip |
|
| 36:07 | coin, there'll be 20,000 heads the and, and then we're gonna call |
|
| 36:11 | a decay. So the rate of is proportional to the amount of parent |
|
| 36:16 | right here. We only got the Astros game, we're gonna get |
|
| 36:21 | . It's all the same stuff. just get more when we have |
|
| 36:23 | So we can write that mathematically as rate of change, the NDT is |
|
| 36:30 | to N right number. We have to time. It's gonna be some |
|
| 36:36 | next to N. And we can that poor proportionality as an equation. |
|
| 36:40 | we toss in this decay constant, lambda, which is going to be |
|
| 36:44 | us something about the probability of a event. If that was a, |
|
| 36:49 | was a, a quarter being this lander would be 0.5. If |
|
| 36:53 | was, you know, for it's gonna be a number of like |
|
| 36:55 | to the minus 10. But it's some number that is uh specific to |
|
| 37:00 | system we're interested in whether it's uranium or flipping quarters or whatever. |
|
| 37:06 | this equation then is something that this the beginning of our ability to tell |
|
| 37:10 | is knowing that the rate of decay proportional to the number we have. |
|
| 37:15 | can rearrange and integrate that equation. we can, we can see that |
|
| 37:19 | can just write that as the natural , the, the, the negative |
|
| 37:23 | the natural log of the number we is is is equal to the, |
|
| 37:29 | proportionality content. Uh the decay constant time. This is what we're |
|
| 37:34 | right? Plus some, some, constant of integration. Um However, |
|
| 37:42 | we take, if we make an here and we take the amount of |
|
| 37:46 | present at the beginning at times if we say that's N zero, |
|
| 37:52 | we can evaluate the constant of, integration and that equals minus log of |
|
| 37:58 | zero. So we can substitute that in. And now we've got the |
|
| 38:02 | a log of N equals N the T times minus log of N |
|
| 38:07 | You can rearrange all that. Do , all the rules of logs do |
|
| 38:14 | and put these, put, put side of the equation race to the |
|
| 38:17 | , you get the ratio of what have today. And the number you |
|
| 38:22 | with N zero is equal from E the minus lambda T. So that's |
|
| 38:29 | . We can measure, we measure in the lab and that's something we |
|
| 38:34 | in the lab. Lambda. We know this already because we've done other |
|
| 38:38 | to tell us what that is. so, gosh, all we need |
|
| 38:41 | know is how many we started That's not something we could measure the |
|
| 38:46 | . So what's the, you that, that, that, that |
|
| 38:49 | , that seems great in theory. how are we gonna use this? |
|
| 38:53 | , we can carry on a little further, we can substitute and we |
|
| 38:56 | , we can, we can uh that equation for either N or N |
|
| 39:02 | . And, and if we do , if we, if we then |
|
| 39:05 | say that now we're gonna involve the , we know that the number of |
|
| 39:11 | , the number of daughters star means . The number of daughters that were |
|
| 39:16 | by radioactive K, it's gonna be to the number of parents that we |
|
| 39:21 | with. Subtracting away from the number parents that we have right. |
|
| 39:26 | This is assuming that this is a system and all we do is we |
|
| 39:30 | a parent, it transforms into a . So, and, and plus |
|
| 39:34 | is always gonna equal and not. now we've got an expression in which |
|
| 39:40 | , which we can get rid of not and not. It's not something |
|
| 39:43 | can know, but we can know is the daughters. We can measure |
|
| 39:48 | in the lab. So we substitute back in here. And now we |
|
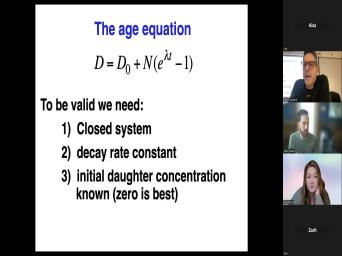
| 39:51 | an equation and substitute all this And now this is it this is |
|
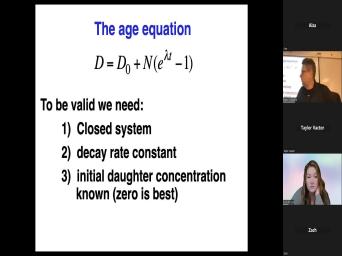
| 39:55 | thing at the bottom here is called age equation in which we say that |
|
| 39:59 | number of daughters is equal to the of daughter we started with. |
|
| 40:05 | excuse me, I, I skipped . I'm gonna go down here. |
|
| 40:09 | , the number of the number of JIC daughters is equal to the number |
|
| 40:14 | parents. We have times Z and minus one. However, um, |
|
| 40:21 | we assume there were no daughters at beginning, then, um, we |
|
| 40:29 | , uh, get rid of T here and do it this way, |
|
| 40:34 | get D equals D, not plus either and the t much one. |
|
| 40:38 | you look at that and you still got a problem there, |
|
| 40:41 | Because we're still being asked, we measure this, we measure the number |
|
| 40:46 | , of parents, we have that is something we measure in the |
|
| 40:49 | D, the number of daughters, can measure that in the lab. |
|
| 40:52 | again, we've just substituted N not DNO. How's that? How's that |
|
| 40:56 | ? Well, we're actually, gonna find a way but this is |
|
| 41:00 | equation D equals D not plus nd lada T. Before we, before |
|
| 41:06 | fix this problem, we will fix problem straight away. I wanna make |
|
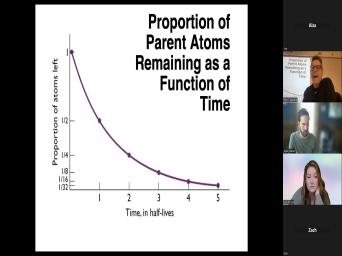
| 41:11 | we understand this concept of half And so we've talked about this |
|
| 41:17 | The half life is something that's The rate of decay is something that's |
|
| 41:22 | because the half life is defined as , the time required for half of |
|
| 41:29 | we have to decay away. And at team one half a after we've |
|
| 41:37 | through one half life, the number daughters and the number of parents should |
|
| 41:41 | exactly, exactly the same because that's have these now, please. |
|
| 41:49 | I'll do it this way. Ok. Thank you. Thank |
|
| 41:52 | Um, so the number of daughters parents should be equal when you um |
|
| 41:59 | you've been through one half life. if we plug in one half life |
|
| 42:03 | T, we can say that D N are the same. So we'll |
|
| 42:07 | put in N here twice and we then rearrange that and we can say |
|
| 42:11 | the half life is then gonna be natural log of two divided by the |
|
| 42:17 | the decay constant or 0.63 0.69 divided the decay constant. We can graphically |
|
| 42:24 | this uh this here. We have uh on the y axis. We |
|
| 42:28 | the proportion of atoms left if you with one. And then we just |
|
| 42:33 | this over time. After one half , we're gonna have half of what |
|
| 42:36 | had. After two, half we're gonna be down to a |
|
| 42:39 | three, half lifes, down to eighth and so forth. And so |
|
| 42:41 | see that this is why I said the rate of decay isn't constant. |
|
| 42:47 | the, what is constant is how it takes to drop by half. |
|
| 42:50 | the half life that doesn't change, is related to this probability, |
|
| 42:55 | in, in whether you're flipping a or you're, you know what's, |
|
| 43:00 | , another physical? Oh, excuse , I'm gonna sneeze. Or maybe |
|
| 43:04 | won't, let's find out. Um, you can flip a quarter |
|
| 43:09 | you can roll one of those, know, Dungeon and dragons 20 sided |
|
| 43:12 | . Right. It's the same idea that in one case, you, |
|
| 43:15 | get, you get the thing half time. In the other case, |
|
| 43:19 | get 1/20 of a time. Uh the case of uranium, it's like |
|
| 43:24 | a billion sided die. You it doesn't happen very often. But |
|
| 43:27 | you have a, if you have trillion of these things in your, |
|
| 43:29 | your, in your zircon, you're them all the time you're having decay |
|
| 43:34 | . Uh This described this, this also describes why it's not a good |
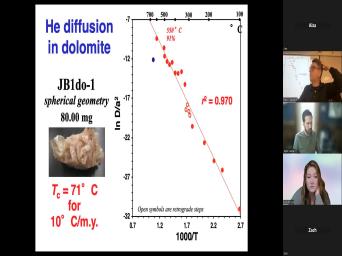
|
| 43:40 | to try and date something after it experienced more than about five half |
|
| 43:47 | Did you see what's happening to this ? The slope of this curve is |
|
| 43:51 | nice and flat here. The and we're gonna have to measure |
|
| 43:56 | this, this, this value here then bring it over to this thing |
|
| 44:00 | read down the, the age and see that as, as we get |
|
| 44:04 | to these really small numbers, any of uncertainty in that number is gonna |
|
| 44:09 | into a huge uncertainty in where it this purple curve. So it's not |
|
| 44:13 | useful to try and date something that's through more than about five half |
|
| 44:17 | Because when you make a measurement in lab, it's very hard to tell |
|
| 44:21 | it's gone through five half lives or half lives. It's just the, |
|
| 44:24 | slope is just so flat that this why we can date. This is |
|
| 44:28 | that carbon 14 dating is probably the system. You're most familiar with carbon |
|
| 44:34 | dating is you read about it in newspaper when they're trying to date |
|
| 44:37 | you know, some archaeological site, good for things that are hundreds to |
|
| 44:41 | few 1000 years old because it has half life of 5700 years. It's |
|
| 44:48 | for dating something more than about 20,000 . If you wanna date something that's |
|
| 44:53 | of years old, you have to something that have half lives that are |
|
| 44:56 | of years old. Um We can this graph as the decay of the |
|
| 45:02 | atoms in the same way as we draw the growth of the daughter |
|
| 45:05 | One is rising, the other is . The, the, the total |
|
| 45:08 | change. Um I said this already I'll skip that. Um So as |
|
| 45:16 | said, the uh some daughter products themselves radioactive, although everything eventually comes |
|
| 45:22 | something stable. So sometimes we can the stuff in the middle and just |
|
| 45:26 | straight to the final daughter product. an example of uranium 238 bunch of |
|
| 45:31 | decays, but it decays down to to a six. Ok. And |
|
| 45:36 | talk more in detail about this when get to uranium dating. OK. |
|
| 45:43 | , um one real big deal here that when it comes to radioactive |
|
| 45:50 | we have to be really strict uniform . Um you know, uniform. |
|
| 45:57 | is this idea that the president is key to the past. If we |
|
| 46:00 | know about today, we know about past, but we know that that's |
|
| 46:03 | strictly true because, you know, we, if we try to apply |
|
| 46:07 | is to all geologic events in the , we'd never say that the dinosaurs |
|
| 46:11 | killed by a meteor because a meteorite falling out of the sky this |
|
| 46:16 | The presence is now, but the experience is, is broader than |
|
| 46:22 | That can't be true for radioactivity. can't say that well, in the |
|
| 46:26 | , uh radioactivity was different. We to uh we have to say that |
|
| 46:29 | is some natural law like water always downhill and uranium always had this proportion |
|
| 46:35 | , of, of, of radioactive . And that's important. And so |
|
| 46:40 | are, you could say it's an that the half lives of the ra |
|
| 46:43 | isotopes are the same as today as were billions of years ago. It's |
|
| 46:46 | an assumption that's been borne out by . Uh One of the ways they |
|
| 46:50 | observe this is go to rocks from moon. The moon is an excellent |
|
| 46:56 | for this thing because the, the and the moon are old and they |
|
| 47:02 | also had this very simple history. What are, what's something that happens |
|
| 47:08 | earth that doesn't happen on the Excuse me, gravity happens on the |
|
| 47:17 | . There's, it's not as, know, but I mean, there's |
|
| 47:20 | gravity, thermal reset of the Well, that's, you're, you're |
|
| 47:29 | the right track there. But why there no thermal reset? I think |
|
| 47:33 | had something, plate tectonics, there's plate tectonics on the moon which would |
|
| 47:37 | to no tectonic burial and getting the reset. I mean, strictly |
|
| 47:41 | if you could drop something down into interior of the moon, it get |
|
| 47:45 | enough, but it doesn't happen because no plate tectonics. Stuff that sitting |
|
| 47:48 | the surface of the moon has been there for a very long time because |
|
| 47:52 | tectonics is over. Now, there's thing that's problematic for us here on |
|
| 47:57 | that doesn't happen on the moon, , it never rains on the |
|
| 48:09 | And this is a wonderful thing for samples, right? Because we don't |
|
| 48:13 | to worry if they've been altered in way. Um First thing you wanna |
|
| 48:16 | when you're doing a lot of geo is get a thin section. Look |
|
| 48:19 | your rock, see if it's you know, if it's been |
|
| 48:22 | then you have to worry about your been screwed up somehow. It's never |
|
| 48:26 | on any of these rocks in the . So we've got these basalts from |
|
| 48:30 | moon. They are old and they , they've never been buried, they've |
|
| 48:34 | been rained on, couldn't ask for better sample. And so these samples |
|
| 48:38 | been brought back and taken to places JSC down the road here and they've |
|
| 48:42 | dated by many different isotopic systems. if the, I if we, |
|
| 48:49 | we had gotten our estimates of the constant wrong or if we had gotten |
|
| 48:54 | right for today, but they had over time, we shouldn't get the |
|
| 48:58 | answer by, by, by doing these different systems, they should be |
|
| 49:02 | . But we, we can date of these rocks by sometimes up to |
|
| 49:05 | different independent systems. And these are that we would predict if we know |
|
| 49:10 | if we know the decay constant, of these answers should be the |
|
| 49:14 | They are the same. Um you know, maybe we'll find some |
|
| 49:18 | to doubt this sometime, but we that there's no major problem. And |
|
| 49:23 | these are the half lives of the we're gonna talk about. Mostly. |
|
| 49:26 | fact, there's only, well, are, these are some geologically interesting |
|
| 49:30 | lives and they vary by a factor 100. You see that the uh |
|
| 49:34 | 235 decays to lead to a seven a half life of 700 million years |
|
| 49:40 | suma 147 decays to su to Excuse me, that's a mistake. |
|
| 49:44 | should be neodymium. 143. Uh change that later. Um This |
|
| 49:52 | um That's actually a mistake that should 143 that has a half life of |
|
| 49:57 | billion years. These are still useful us. Um However, most of |
|
| 50:03 | things we're gonna talk about involve the of potassium and uranium. These are |
|
| 50:08 | of illustrative but not used of But most geology can be understood by |
|
| 50:13 | decay of potassium and uranium. Potassium uranium are abundant enough in rocks and |
|
| 50:19 | to be useful. And they have half lives that range from 0.7 to |
|
| 50:23 | billion years, which means that they've around about the right amount of time |
|
| 50:29 | in excuse me, thorium um has half life of a 12 billion years |
|
| 50:38 | we'll talk about that yet. It didn't have room for thorium on this |
|
| 50:41 | . But it's a, it's a . It's got a 12, |
|
| 50:43 | it's 12 billion. So what we happens is the, and that should |
|
| 50:49 | that that shouldn't say rate of It should say probability of decay, |
|
| 50:53 | probability of decay over time hasn't changed it's isotope A B or AC just |
|
| 51:00 | a graph, whatever the probability is . Flip A coin, 50%. |
|
| 51:04 | , that coin was a 50% coin billion years ago. Um We know |
|
| 51:09 | didn't happen that if that, if been any sort of variation in the |
|
| 51:13 | of things, they didn't vary independently that these things are, are changing |
|
| 51:17 | again, this would show up in uh rocks from the moon or even |
|
| 51:24 | rocks here on earth rol lights from, you know, 20 million |
|
| 51:28 | ago, we date them by different to get the same answer. So |
|
| 51:35 | lots of ways then have been, been sorted out to try and understand |
|
| 51:39 | rocks in the age of the One of the, one of |
|
| 51:43 | one of the uh famous ways um um Lord Kelvin or William Thompson in |
|
| 51:51 | 1870 published an an estimate for the of the earth. He said that |
|
| 51:57 | we know how big the earth We know how uh uh we know |
|
| 52:02 | big it is and we can estimate original temperature. He estimated the temperature |
|
| 52:08 | molten iron and, and by he went down into AAA coal mine |
|
| 52:15 | Wales and measured the temperature down one below the surface. And with that |
|
| 52:20 | , he was able to come up some stuff and say, well, |
|
| 52:22 | earth is this big, it started this hot, it's this thermal uh |
|
| 52:27 | geothermal gradient today. That means the is somewhere between 30 100 million years |
|
| 52:33 | . But the problem was is that didn't know about radioactivity. Um And |
|
| 52:40 | he didn't know about radio activity is you've noticed in all of these equations |
|
| 52:43 | I showed you this decays to this Q on the end there's energy. |
|
| 52:48 | And so Kelvin got the edge of earth way, way underdone because he |
|
| 52:52 | appreciate that the earth was cooling slower he imagined because it was making its |
|
| 52:56 | heat. Well, that's, you , you can redo his equations and |
|
| 53:01 | it out how the earth, how the earth is now by that |
|
| 53:04 | But the better way is to go and date rock straight away. And |
|
| 53:09 | the discovery of radioactivity about 100 and years ago by, you know, |
|
| 53:14 | folks you've probably heard of in physics Beal Kri Rutherford, it provided a |
|
| 53:20 | of heat to override this mistake that made in his calculations. But then |
|
| 53:24 | also provides the basis for any of quantitative estimates for ages and rocks. |
|
| 53:29 | is how we could say the dinosaurs extinct 66 million years ago or this |
|
| 53:35 | in Trinidad is, you know, million years old. Um I'm gonna |
|
| 53:42 | that, skip that. So, well, anyway, we know we |
|
| 53:50 | from looking at some rocks at the is the edge of the earth is |
|
| 53:53 | billion years. Um All right, questions about that little derivation should be |
|
| 54:02 | straightforward. We've got an age Um And with that, we |
|
| 54:06 | if we measure things in the we can figure out an age of |
|
| 54:11 | . And as I said, we this, this diagram here before |
|
| 54:15 | uh we know that, you we've, we've made changes in the |
|
| 54:18 | of important geologic boundaries over time because gotten better outcrops and better technology I |
|
| 54:27 | um I just know that you, I didn't say it, but it's |
|
| 54:35 | excellent question. Um In some we can me, it has been |
|
| 54:41 | directly. Um And, and, , and there was actually an experiment |
|
| 54:45 | was done to, to understand the of rubidium 87. They actually uh |
|
| 54:52 | refined a bottle of rubidium, you , this, they just got it |
|
| 54:57 | that this rubidium had, was just pure bottle of rubidium. And they |
|
| 55:01 | how much strontium was in there. little. But they, it's a |
|
| 55:04 | , big pile of rubidium and a , little bitty bit of contaminants of |
|
| 55:08 | . But they measured that and then put it on the shelf for 40 |
|
| 55:14 | and then they measure it again and got more staunch in it than it |
|
| 55:18 | 40 years ago. And that sounds of crude, but it worked. |
|
| 55:23 | the reason and they figure, and , they knew that the half |
|
| 55:26 | excuse me. And with this, can calculate the half life. |
|
| 55:29 | it turns out the half life was billion years. So you might |
|
| 55:34 | well, how are you gonna be to measure that tiny little change if |
|
| 55:37 | half life is 47 billion years? did we get anything in just 40 |
|
| 55:42 | ? And the answer is because we a pound of rubidium here. |
|
| 55:46 | the number of decays is dependent on many we have. If you actually |
|
| 55:51 | a huge hunk of rubidium, a or a kilogram, I don't know |
|
| 55:54 | much they had, they had a that then even if the half life |
|
| 55:59 | billions of years, if you have kilogram of that stuff, that means |
|
| 56:05 | of these things are sitting there ready decay and in 40 years, a |
|
| 56:10 | million of them will decay and that's measurable thing. And so with |
|
| 56:17 | they got the half life of rubidium there. They start looking at geologic |
|
| 56:23 | that, that have every expectation that should get the same answer no matter |
|
| 56:27 | system we use. Usually volcanic If you know the half life of |
|
| 56:32 | system, you can date that rock that system and then you date the |
|
| 56:35 | , you date you, you do chemical analysis for another system and then |
|
| 56:40 | take the age as given from the one, you know, and calculate |
|
| 56:43 | half life that way. Did you that Taylor? OK. So that's |
|
| 56:48 | or less how it's done. A of them have been, have been |
|
| 56:51 | from first principles and then the rest them are basically relative to those |
|
| 56:57 | So that's a good question. That's . Um, you guys wanna take |
|
| 57:05 | brief break in an hour. we usually go till how late on |
|
| 57:10 | day? Five, right? And tomorrow we start at 830. Is |
|
| 57:13 | correct? All right. Let's uh, let's give my, my |
|
| 57:18 | to 10 minutes because we're at we're at a good stopping point |
|
| 57:24 | So we'll come back in 10 Ok. So we got an age |
|
| 57:36 | and, but to really have this , make any sense. We need |
|
| 57:40 | three things to be true. We've talked about the decayed constant thing. |
|
| 57:45 | that's already been sorted out. And we talk about rubidium strontium in the |
|
| 57:51 | section, we will address this question how many daughter products, how many |
|
| 57:57 | there are to begin with. In cases, we can geo chemically, |
|
| 58:00 | can say there aren't any daughters to with. Like potassium argon, the |
|
| 58:05 | potassium decay to argon. But because is a noble gas, it doesn't |
|
| 58:10 | parts of minerals. So we can pretty much sure that when we measure |
|
| 58:15 | , it was because of radioactive it wasn't that begin with. In |
|
| 58:18 | cases, it's more complicated than We'll get to that in a minute |
|
| 58:22 | in an hour. Uh But what gonna talk about. Next is this |
|
| 58:27 | of a closed system? Um, we mean by a closed system is |
|
| 58:34 | we have no loss or gain of or daughters except for in situ radioactive |
|
| 58:41 | right here in our rock or our , the parent decays to a |
|
| 58:45 | That's the only thing that happens and does. So, within the context |
|
| 58:49 | this system, we're talking about, , the system would be the mineral |
|
| 58:55 | gonna look at minerals. So this F bar or zircon or whatever it |
|
| 59:02 | . So when we talk about open behavior, the chief concern in these |
|
| 59:07 | is going to be the loss of daughters. Um Generally, don't worry |
|
| 59:12 | loss of parents. Um And that's because daughters were, were created an |
|
| 59:19 | , they were not a part of original mineral. And so when they |
|
| 59:22 | formed in there, um they may be happy in there. You |
|
| 59:26 | the like potassium decays to argon, is now in this mineral, but |
|
| 59:30 | not a part of the lattice, not there in any strong way. |
|
| 59:37 | now, of course, you can an open system in which you add |
|
| 59:40 | uranium. Uh But we're really, just gonna ignore that for the |
|
| 59:44 | we're gonna talk about open system as loss of daughter products. Um And |
|
| 59:50 | the loss of data products due to particular phenomenon called diffusion within the |
|
| 59:56 | Um And we'll talk about that in minute. But what this then means |
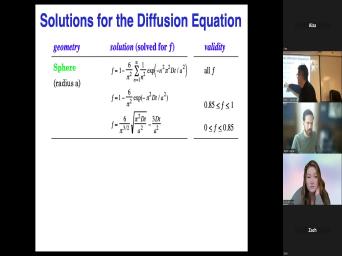
|
| 60:01 | that when we have a geo chronological , this spelt bar is 10 million |
|
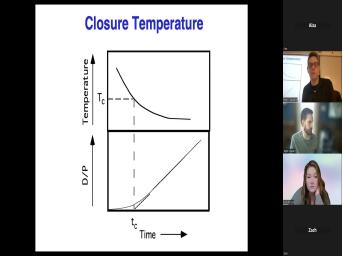
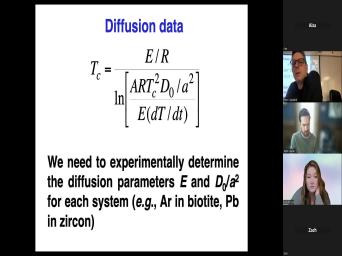
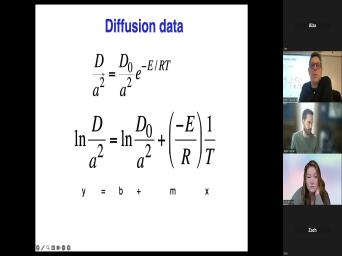
| 60:06 | old. What that means then is was the last time the beds bar |
|
| 60:12 | at the temperature at which this system a closed system. Um Sometimes that's |
|
| 60:21 | . Sometimes there are geologic complications that have to keep in mind. But |
|
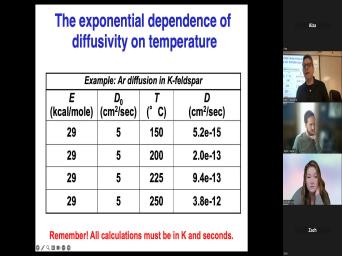
| 60:25 | we get into the geology, we'll about more of the physics. So |
|
| 60:31 | is defined as a thermally activated which means that it, the rate |
|
| 60:36 | as you increase the temperature. Um indeed, as we'll show in a |
|
| 60:40 | , it's not, it doesn't just a little bit, it increases exponentially |
|
| 60:44 | , the rate of something it has in the exponents, the, |
|
| 60:48 | the, the rate equals something times to the T. So, um |
|
| 60:53 | change temperature a little bit, the can change a whole lot. The |
|
| 60:57 | thing about diffusion is it's just totally . If, if, if uh |
|
| 61:01 | move jiggling around, it will jiggle a particular way, but that's, |
|
| 61:06 | randomly directed. Uh And so it look like, I mean, |
|
| 61:11 | it is the case that generally what have a system in which we |
|
| 61:14 | we, we have a diffusion situation which we move from high concentration to |
|
| 61:19 | concentration. That's not because the you know, the things that are |
|
| 61:22 | high concentration we're seeking out that area there. I mean, basically when |
|
| 61:27 | put, you know, you put in your iced tea, you |
|
| 61:30 | your sugar becomes after if you, you stir that sugar up, pretty |
|
| 61:34 | that tea that sh that tea is , the same sweet because random diffusion |
|
| 61:40 | made it. So uh what we in our situation is we have, |
|
| 61:46 | have an the iced tea. If take that analogy, the the sugar |
|
| 61:50 | get out of the glass. You , the, the thing that we're |
|
| 61:53 | at this thing that's diffusing it, only diffusions randomly within our system, |
|
| 61:58 | under the right conditions, it can right out. And I'm gonna illustrate |
|
| 62:03 | with a silly example. It's a , very uh crude example, but |
|
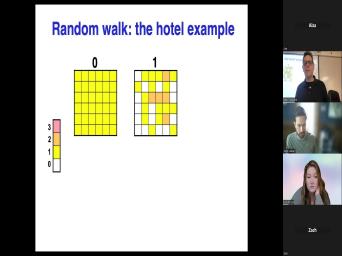
| 62:08 | hope it works out. Ok. And we're gonna imagine a hotel, |
|
| 62:14 | odd hotel that has 36 rooms, no hallways and on the, each |
|
| 62:19 | of these rooms has a door on four walls. Um And what we're |
|
| 62:26 | do is start in this ho this with one person in each room. |
|
| 62:29 | that's color coded here. Yellow means . And then we're gonna have each |
|
| 62:34 | of these people pick a random number they'll move north, south, east |
|
| 62:38 | west, depending on what they which is totally random. And the |
|
| 62:43 | are, if you move into another , you stay. But if you |
|
| 62:46 | to the outside of the hotel, gone and you can't come back |
|
| 62:49 | And this is, this is analogous what we think happens in diffusion and |
|
| 62:53 | . Once a daughter product diffuses it's now diffusing out there and it's |
|
| 62:57 | different story and it's not coming back . So we start this by, |
|
| 63:02 | know, just picking these random And now we have a situation here |
|
| 63:06 | we have several of the rooms on outside, have nobody in them because |
|
| 63:11 | all, all of these, all these rooms, you know, had |
|
| 63:15 | , the, the the random walk to walk outside. Uh Whereas in |
|
| 63:20 | ones in the middle actually got more in them because the, the random |
|
| 63:23 | pushed two people in the same But if we do this long |
|
| 63:27 | we will see that eventually we get situation in which we have a high |
|
| 63:33 | in the center and very little on outside. And of course, this |
|
| 63:38 | a ridiculously a coarse example with only rooms and five time steps. But |
|
| 63:43 | did this in Excel many years If you wanna do it fancier, |
|
| 63:46 | could figure out some sort of Python to make it beautiful, but it's |
|
| 63:50 | same concept. Um And we can look at this in terms of its |
|
| 63:56 | , but also in terms of its amount, you see, we started |
|
| 63:59 | with 36 then we move to 26 25 we get down to 21 and |
|
| 64:04 | is all because of just moving things randomly about. So that's diffusion, |
|
| 64:11 | thermally activated diffusion would just go faster it was hotter. So we |
|
| 64:15 | we could move from step zero to five, very fast if we were |
|
| 64:19 | a high temperature, very slowly, we were at low temperature. And |
|
| 64:24 | at, at high temperatures, things , you know, if, if |
|
| 64:27 | let this go a little bit further you know, to basically simulate at |
|
| 64:31 | temperature, we go through more time . We do this a few more |
|
| 64:35 | . You'll see that the hotel will empty out. And it's not hard |
|
| 64:38 | imagine. We do this a few times. It's just it, it |
|
| 64:41 | away. Um And that's the concept closure temperature. If you, if |
|
| 64:46 | turn the temperature up high enough, things are going out all the |
|
| 64:50 | Uh But if the temperature is very , you may, you know, |
|
| 64:54 | may the, the the the rate which you change rooms becomes essentially |
|
| 64:59 | And this is why, for we can hold a metamorphic rock in |
|
| 65:02 | hand, metamorphic rocks are formed down , right? They're not formed at |
|
| 65:06 | surface. You take a shale and put it down, it becomes a |
|
| 65:10 | , it's more it's stable down These minerals form, it's a |
|
| 65:13 | Why is it that a shift doesn't it shift comes back to the |
|
| 65:16 | it doesn't turn back into a It's because the, it's, it's |
|
| 65:21 | shifts are not stable here at the surface, they don't form at the |
|
| 65:24 | surface, but they can sit here the earth surface because the, the |
|
| 65:28 | at which they are transforming back to stable stuff is exponentially dependent on |
|
| 65:34 | And when you drop that temperature down surface temperature, you drop the rate |
|
| 65:38 | reaction literally trillions of times. So it's unhappy. And if you let |
|
| 65:44 | sit here for another 10 trillion it might turn back into a |
|
| 65:48 | So temperature is very important. So gonna put some formality to this so |
|
| 65:55 | we can really think about the con , the the things which govern uh |
|
| 66:00 | temperature, we wanna do that. so that we can, we need |
|
| 66:04 | have this formality so that when we talking about choosing samples and interpreting data |
|
| 66:10 | our geologic context, we know what some of the pitfalls and concerns. |
|
| 66:15 | so we're gonna start, we're gonna this thing called the diffusion equation. |
|
| 66:19 | from that, we will get the temperature equation. And we're gonna start |
|
| 66:24 | imagining this simple thing in which we a one dimensional rod which I've sort |
|
| 66:29 | shown a two dimensional rod, but this one dimensional rod in which energy |
|
| 66:33 | only travel in one direction. And is thermal energy is throwing flowing past |
|
| 66:38 | points A and B, we can that the total heat energy in the |
|
| 66:44 | between A and B is some integration this energy function between, you |
|
| 66:49 | integrate from A to B this function which is a function of distance and |
|
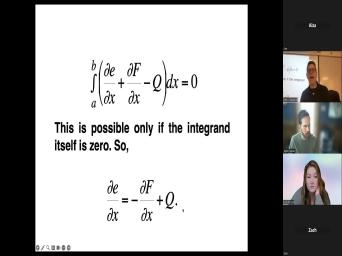
| 66:55 | . OK. Now, because we're gonna describe conservation of energy, |
|
| 67:02 | gonna say that the rate of change heat energy is equal to the heat |
|
| 67:09 | flowing across the boundaries per unit time whatever is generated inside this rod. |
|
| 67:16 | we can write that mathematically to say . Now we're talking about the rate |
|
| 67:20 | change. So that's this DTD over business, the rate of change of |
|
| 67:25 | thing. And this just be described the heat flowing across the boundaries. |
|
| 67:30 | the, the flux at A as function of time minus the flux at |
|
| 67:36 | as a function of time plus this Q which is our internal heat |
|
| 67:42 | Um So that's just taken that first and written it as this thing. |
|
| 67:50 | , if we, if we just a little simple fundamental theorem of calculus |
|
| 67:56 | , we can uh know that this is equal to the derivative of these |
|
| 68:04 | . And by, and we can that to say that now we have |
|
| 68:07 | the interval from A to B is to the, the change in energy |
|
| 68:11 | respect to distance plus the change in with respect to distance minus whatever is |
|
| 68:19 | generated inside relative to distance. the good news is, is that |
|
| 68:25 | up in there that that equation is possible if the integrand is itself |
|
| 68:31 | So we can get rid of all integration. And just say that |
|
| 68:34 | the change in energy with respect to is equal to the change in flux |
|
| 68:38 | respect to distance plus Q, there's minus sign in there just because of |
|
| 68:43 | , the way in which we're defining and B. Now we usually describe |
|
| 68:50 | by their temperature, not their thermal , which is what we were really |
|
| 68:55 | back there. So to convert into , we have to come up with |
|
| 68:59 | things called specific heat and density. that's pretty straightforward. Um We know |
|
| 69:05 | the ma the, the, the mass, excuse me, |
|
| 69:08 | the thermal diffusivity is then gonna be to the, the specific heat, |
|
| 69:13 | is a function of X times the , which is a function of X |
|
| 69:16 | the temperature, which is a function X and time. And so we |
|
| 69:21 | that back in there. Now, got this equation in which all we |
|
| 69:24 | all we have now is the specific and the density which we're just gonna |
|
| 69:29 | are actually constants. And we, we see that we've got an expression |
|
| 69:34 | has temperature time and the flux of energy. So we're getting, we're |
|
| 69:38 | somewhere. So now we get, we carry on, let's just consider |
|
| 69:43 | of these questions, how does heat flow? And by the way, |
|
| 69:48 | talking about heat energy. Now, of this stuff that we're talking |
|
| 69:53 | the mathematics and the philosophy of all this applies, whether we're talking |
|
| 69:57 | temperature or sugar in your iced tea argon in your F bar or lead |
|
| 70:03 | your zircon. It's all about Heat heat is just an easier thing |
|
| 70:07 | think of. You know, we can, we know heat radiating |
|
| 70:10 | a fire or an oven or a stove. But the same concept, |
|
| 70:15 | same ideas will be transported. When talk, we stop talking about heat |
|
| 70:18 | start talking about daughter products. This is easier to think about in terms |
|
| 70:23 | heat, but it's no different, concept. So let's just, you |
|
| 70:27 | , if some things we can say heat energy, we know for |
|
| 70:32 | that if the temperature is constant, no transfer of energy, we know |
|
| 70:37 | if there are temperature differences, the energy is gonna go from the hot |
|
| 70:40 | the cold. You pick up a , a hot pan, your hand |
|
| 70:43 | burned. Not the other way around greater the delta t, the greater |
|
| 70:48 | heat flow. That's why it's a hot pan. You'll notice immediately and |
|
| 70:54 | flow of heat energy will vary for materials. You pick up a hot |
|
| 70:58 | of wood or a hot piece of , the iron will scald, you |
|
| 71:01 | because it transfers heat better. So are things we can just easily say |
|
| 71:06 | the world. And we can transfer into a mathematical equation in which we |
|
| 71:12 | that the flux of something is gonna equal to the, the thermal, |
|
| 71:19 | flux of thermal energy. Uh excuse , the flux is the of thermal |
|
| 71:24 | and ka is the thermal conductivity and is the change of temperature with respect |
|
| 71:30 | distance. So, um now we've an equation that describes this stuff, |
|
| 71:36 | energy passing through our material. And we have to all, all we |
|
| 71:40 | basically say that this is all dependent this characteristic K dot Which is just |
|
| 71:45 | material property. Um So we that we, that, that we've |
|
| 71:51 | this to get F we can So we've got this equation up |
|
| 71:57 | That's not something we like to deal . Flux is flux is a thing |
|
| 72:00 | changing. But so we can substitute thing. We, we, |
|
| 72:04 | we, uh we, we drive F into here and if we |
|
| 72:09 | now, as I said, we're gonna assume that the, we're gonna |
|
| 72:12 | that we're dealing with one thing, iron or it's feldspar. So we're |
|
| 72:16 | gonna say that the specific heat and density are a function of distance. |
|
| 72:20 | are just a, a material Feldspar has this density. And so |
|
| 72:25 | we can rearrange those things and get of those guys. And and then |
|
| 72:30 | it finally to say that the, change in temperature with respect to time |
|
| 72:37 | equal to the second derivative temperature with to distance uh mediated by this deep |
|
| 72:44 | , which is the diffusivity, which just our, our thermal conductivity divided |
|
| 72:49 | our density and our, our uh specific heat. This is known as |
|
| 72:57 | second law, the diffusion equation. it's, it's fundamental and you can |
|
| 73:02 | that it's, it's, it's, pretty straightforward to go from just imagining |
|
| 73:05 | one dimensional rod and, and just a few things about heat. And |
|
| 73:09 | we've got this, this, this thing. So what's that good |
|
| 73:13 | ? 00 I should say that this assumes that the diffusivity is the |
|
| 73:19 | in all directions. Um But in meteorology, as well as |
|
| 73:25 | we don't have to assume that these are the same in one direction. |
|
| 73:28 | we can generalize this three dimensions. we can have a diffusivity in the |
|
| 73:32 | direction and Y direction we might wanna that. And as I said, |
|
| 73:37 | equation can, is just as valid the diffusion of mass or the concentration |
|
| 73:43 | mass as thermal energy. So that's nice about this is we derived it |
|
| 73:47 | thermal energy, but we're now we're apply it to things in our |
|
| 73:55 | Now, one more thing that's interesting helpful is that although d the diffusivity |
|
| 74:01 | expressed as a constant in this, this uh equation, it is itself |
|
| 74:07 | on temperature. And this is something pretty straightforward. We talked about earlier |
|
| 74:11 | the, that this these processes move if it's hotter. So, so |
|
| 74:19 | got a, we've got an expression that says the diffusivity is actually |
|
| 74:23 | I should do that. The diffusivity actually related to this D not, |
|
| 74:28 | is just basically the diffusivity at infinite . And then mediated by these things |
|
| 74:33 | the natural, natural ee is E big E is something called an activation |
|
| 74:40 | . R is the gas constant and is temperature. So this activation energy |
|
| 74:45 | a, again a, a function the material. It might be harder |
|
| 74:49 | diffuse through zircon than it is through , for example. Um And so |
|
| 74:58 | substitute this. So also what is and activation energy is the energy barrier |
|
| 75:06 | the reactants must overcome for a reaction proceed. Um If you have |
|
| 75:12 | if you had a car that was in gear, but on a flat |
|
| 75:17 | , you could get it going, could get it rolling. But the |
|
| 75:20 | , the more people in the the higher the activation energy you gotta |
|
| 75:23 | , you gotta really push it to it going. Once it rolls, |
|
| 75:26 | can roll. But the activation energy , of a big car is much |
|
| 75:31 | than the activation energy of sit of guy sitting on a bicycle, you |
|
| 75:34 | move that easier. So that's, the concept of activation energy. And |
|
| 75:41 | to show you the uh how, , how sensitive this is to, |
|
| 75:45 | temperature. I just gave you some that are not, they're good |
|
| 75:51 | It's not too important that we look a lot of the details. But |
|
| 75:53 | we just put in some numbers here diffusion of argon in feldspar, which |
|
| 75:58 | talk more about tomorrow. But just an example, if we pick a |
|
| 76:01 | of 29 kilocalories per mole and ad of five, those are just perfectly |
|
| 76:06 | numbers. But, and we just , let's start with a temperature of |
|
| 76:10 | and 50 degrees. We go through calculation, this calculation here and we |
|
| 76:16 | a, we get a value of at that temperature D is then a |
|
| 76:19 | of temperature D at 100 and 50 is five times seven minus 15 centimeters |
|
| 76:24 | per second. That's, but look happens when we change the temperature to |
|
| 76:31 | degrees, 100 degree change. Look happened to the te, the |
|
| 76:36 | It went from five times 10 to minus 15 to 4 times 10 to |
|
| 76:40 | 12. That's a factor of 1000 ? We've made it go 1000 times |
|
| 76:48 | by increasing the temperature by 100 If we decrease it, if we |
|
| 76:52 | it another 100 degrees, it'll be 1000 another 1000 another 1000. So |
|
| 76:57 | explains why there'll be some, there'll some temperatures at which is going super |
|
| 77:02 | or super slow. Um Oh, a factor of 713 fine. All |
|
| 77:08 | . Uh I'm gonna, I'm, , I'm also, I'm gonna zip |
|
| 77:11 | these other examples. Um All So we're almost through with some of |
|
| 77:19 | equation stuff. Um But now that got this, this, this, |
|
| 77:24 | uh relationship between temperature distance and time can, uh we can refine a |
|
| 77:32 | bit. Um any of you, all taken calculus, right? If |
|
| 77:39 | take differential equations, any of no, not important. But this |
|
| 77:44 | what's called a differential equation. And of the ways you deal around this |
|
| 77:52 | that you can make certain assumptions about geometries or initial conditions and things get |
|
| 77:58 | lot easier. And so what we're do here is make a few assumptions |
|
| 78:04 | the shape of the thing we're diffusing , it can be a sphere, |
|
| 78:08 | can be a cue, it can whatever. Uh And when we make |
|
| 78:13 | assumptions, this happens and let me well, let me just go back |
|
| 78:20 | here. We have an equation that t temperature time, diffusivity and |
|
| 78:27 | We make some assumptions about the geometry things. We can get equations that |
|
| 78:31 | like this. I know you can't this. I know you don't need |
|
| 78:34 | remember this, but I just want show you that there are examples for |
|
| 78:37 | sphere, the infinite cylinder, the sheet and the cube. And what |
|
| 78:40 | can do is we, we can , so we can, we can |
|
| 78:44 | this equation two ways one in which can solve for this parameter D on |
|
| 78:49 | squared or I should go back and the A, we're gonna, we're |
|
| 78:54 | introduce a couple new terms. A is gonna be a, a |
|
| 78:58 | characteristic A will be the radius of sphere or the side of the cube |
|
| 79:03 | the radius of the cylinder or the of the slab. So by, |
|
| 79:07 | introducing a geometry, we can then relative to those things, we can |
|
| 79:12 | assumptions. The other, the the other variable we're gonna introduce is |
|
| 79:17 | variable F and F is if we , if we d if we start |
|
| 79:23 | diffusion action F is the fraction of stuff we started with that we still |
|
| 79:30 | . So we start with one and you were diffusing and diffusing and diffusing |
|
| 79:34 | if F, if you got rid all of your diffusion F would go |
|
| 79:37 | to zero. So I'm just gonna in, in here on the equations |
|
| 79:42 | uh the sphere. The simplest example just say that these are, these |
|
| 79:47 | the results of some mathematicians working these out. And the thing is is |
|
| 79:51 | what we can do is write equations F which include now things that we |
|
| 79:57 | measure the diffusivity. The temperature and , the, the, the |
|
| 80:06 | And so, um, let me , we're gonna pay attention to this |
|
| 80:13 | . Oh, well, wait a . Uh, yeah, we'll pay |
|
| 80:18 | to this equation in a minute. with that information, we're gonna be |
|
| 80:23 | to hone in on this thing that trying to figure out, gonna, |
|
| 80:28 | formally define this thing called closure So let's just define it. In |
|
| 80:33 | words, closure temperature of a system defined as the temperature at which the |
|
| 80:39 | and loss of a particular species is . We've got both retention and loss |
|
| 80:44 | on here because we're talking about something growing. This is a radioactive |
|
| 80:49 | We, we've got parents which are to daughters. If we were well |
|
| 80:54 | the closure temperature, the daughter would stay there. If we were well |
|
| 80:59 | the closer temperature, the daughter would leave, but we're always gonna be |
|
| 81:02 | a new one. Eventually, this is gonna decay away. So the |
|
| 81:07 | temperature is that temperature in which the of new ones by the radioactivity of |
|
| 81:12 | thing of the dog of the parent balanced by the loss of things. |
|
| 81:17 | it's by the time we make a one that, that, that, |
|
| 81:19 | one we made in the, in previous step has diffused out of the |
|
| 81:23 | , it's hot enough that it diffuses the same time that it takes to |
|
| 81:27 | a new one. That's the closure . In practice, this closure temperature |
|
| 81:33 | the temperature of the system at the represented by its parent age. We |
|
| 81:40 | parents and daughters in a thing and get an age of 100 million |
|
| 81:46 | 100 million years ago. This this belts bar, this zircon was |
|
| 81:52 | , that temperature. That's what it . So that's that, that, |
|
| 81:57 | means that when we interpret our geo age, we really need to talk |
|
| 82:03 | all of these ages as apparent This is what it appears to |
|
| 82:07 | but we have to understand it in of it being that it was 100 |
|
| 82:10 | years ago, it was at the temperature. Well, that's fine. |
|
| 82:16 | the next thing we're gonna have to is figure out what that temperature |
|
| 82:19 | And if we just say at the doesn't help like saying, it's like |
|
| 82:24 | I was born in my hometown. , and yeah, everybody was, |
|
| 82:28 | if you wanna sort of track your , you have to define what that |
|
| 82:32 | is. Uh So we're gonna, gonna go through some formal formal uh |
|
| 82:39 | in which we can figure out what closure temperature is for different systems. |
|
| 82:47 | By the way, this temperatures, thing is not restricted to geo |
|
| 82:52 | other disciplines have it in particular uh mats, you know, when we |
|
| 82:57 | about dating something or figuring out the magnetic pole or something. Uh You |
|
| 83:03 | heat up AAA thing and it'll lose magnetism magnetism, but it doesn't just |
|
| 83:09 | its magnetism like that. It has , it has the same concept that |
|
| 83:13 | see here. OK. So we can describe closure temperature this |
|
| 83:18 | We, we've described it in let's describe it in, in uh |
|
| 83:23 | . We've got two graphs here in we've got time going across the bottom |
|
| 83:28 | on the top one here, we've temperature rising up as we go |
|
| 83:31 | And in this graph here, we're have the ratio of daughters to parents |
|
| 83:35 | will be rising. So if we it, you know, if we |
|
| 83:40 | a cooling history, that is like this monotonically cooling, just always |
|
| 83:45 | cooler at some point, we will hot enough so that the daughter to |
|
| 83:51 | ratio is essentially zero hot, you . So the diffusivity is so high |
|
| 83:56 | make a new parent. Yep, goes out. So, although |
|
| 84:00 | you know, and, and this be zero for a for a very |
|
| 84:03 | time if the thing stays hotter than closure temperature, if the closure temperature |
|
| 84:07 | very low. For example, the temperature is 100 degrees like it is |
|
| 84:12 | fishing tracks. If you've got a that has been buried to below 100 |
|
| 84:18 | or hotter than 100 degrees, that crystal will stay at apparent zero degrees |
|
| 84:25 | long as you're above 100 degrees. another example, would be a granite |
|
| 84:31 | below the surface and it stays down for a long time. If you |
|
| 84:36 | a closure temperature, that's only 100 . If you were to, if |
|
| 84:39 | were to drill down to a granite five kilometers below the surface and, |
|
| 84:43 | sample that granite, you bring it up, you date some of those |
|
| 84:46 | , they would give you a date zero because they were very recently too |
|
| 84:51 | to retain their, their daughters. eventually it starts to cool down whether |
|
| 84:56 | a, you know, granite that's uplifted or a basin that's being |
|
| 84:59 | Somehow, you're cooling your sample. you'll get to a situation in which |
|
| 85:05 | rate of the, the daughter the, the, the, the |
|
| 85:08 | to parent ratio begins to rise a bit. It rise slowly here because |
|
| 85:12 | still pretty hot. It's still hot that we're losing pretty fast, but |
|
| 85:16 | not losing them at the same rate we were before. And when we |
|
| 85:20 | to this temperature here, that's the at which we're losing them as fast |
|
| 85:26 | we're making them. But as we down a little bit colder, we |
|
| 85:32 | to increase the rate of retention until get to some temperature. Well, |
|
| 85:36 | below this temperature here and at that , the daughter to parent ratio will |
|
| 85:42 | increase at essentially a straight line, will be a line proportional to |
|
| 85:47 | the K constant of the system we're in. And if we were to |
|
| 85:53 | measure that system millions of years we'll get a number. And you |
|
| 86:00 | see that this curve kind of is, is uh is flat and |
|
| 86:05 | deepens up. If we measure millions years later, this curve essentially points |
|
| 86:12 | down to this time. That's the we will get by measuring the |
|
| 86:17 | And excuse me, that's the time get by measuring the daughters and |
|
| 86:22 | And we can read that up and the closure temperature. So graphically, |
|
| 86:26 | what closure temperature means. The time which the time the temperature where you |
|
| 86:31 | at, when we get the time calculate by measuring backwards this way. |
|
| 86:40 | . Now, to uh to, , to add this bit of calculation |
|
| 86:46 | figuring out what each individual closure temperature . We're gonna imagine uh cooling in |
|
| 86:51 | situation in which we're gonna start by on either side of the closure |
|
| 86:57 | we're gonna say consider cooling over intervals at closure temperature where the definity drops |
|
| 87:04 | a factor of heat. And of , we, we picked e because |
|
| 87:08 | gonna be able to cancel it out on. And to put it another |
|
| 87:12 | , we know that the diff the at our high temperature or our low |
|
| 87:18 | divided by our diffusivity at our high , the ratio of those two things |
|
| 87:22 | equal to e we can expand those out because we know that the diffusivity |
|
| 87:27 | , is dependent on temperature from that we saw before. So we've got |
|
| 87:31 | of this. He's equal to But what good is that? |
|
| 87:37 | we can uh oh What am I ? Oh, we just simplify this |
|
| 87:50 | by, by the, you simple algebra, we can simple that |
|
| 87:53 | , they get that out and we that e equals all of that raised |
|
| 87:58 | the exile And then we take the of both sides and we've got one |
|
| 88:04 | all of that there, right? . What good is that? |
|
| 88:06 | We're getting someplace we, this, business, right? In here, |
|
| 88:13 | over T one minus one over T . We can approximate that this way |
|
| 88:19 | two minus T one divided by T , you know, we, we |
|
| 88:22 | do that. Why are we gonna that? Well, because with the |
|
| 88:26 | we've defined this, we know that one plus T 2/2, that's the |
|
| 88:32 | of this thing. And we've already that average to be the closure |
|
| 88:36 | So we've introduced, we, we've our closure temperature here. We also |
|
| 88:41 | that T two minus T one is another way of saying delta T |
|
| 88:46 | and this seems like an odd but it's gonna help, we're gonna |
|
| 88:49 | delta temperature into delta time multiplied by rate of time rate of change of |
|
| 88:57 | with respect to time. And so that's the case, all of |
|
| 89:04 | it's gonna be substituted with this. so we're gonna get that equation. |
|
| 89:08 | here, now we have an equation has the activation energy, the cooling |
|
| 89:14 | , the change in time, the constant and our closure temperature, we've |
|
| 89:22 | our closure temperature in our equation. , this is good news. Um |
|
| 89:29 | gonna use those equations I showed you and we're gonna assume from the moment |
|
| 89:34 | we're gonna talk about a, a geometry because that's nice and simple. |
|
| 89:38 | indeed, we're gonna ignore this second of this thing because it really doesn't |
|
| 89:42 | , add much to the uh to situation. And we're furthermore gonna notice |
|
| 89:49 | at, we've, we've defined this at the closure temperature at the closure |
|
| 89:54 | , we know that F equals zero is the place where we balance |
|
| 89:58 | we're gonna be equal loss. And the definition of F, we've lost |
|
| 90:02 | . So at this point, we plug in the half and then |
|
| 90:06 | so that's just a bunch of numbers . And we can, uh we |
|
| 90:10 | , we can say that delta T one over F, all of |
|
| 90:14 | the 0.5 and the six and the to the three halves, all |
|
| 90:18 | And there's another pi squared in there becomes delta T equals 1/55 divided by |
|
| 90:25 | divided by the A member A is distance. So substitute that in there |
|
| 90:34 | we get that equation. And then finally that our diffusivity is dependent on |
|
| 90:41 | , we can substitute that back in . And all of that gets us |
|
| 90:45 | , this equation. And I hope wasn't too painful, but we can |
|
| 90:49 | see where that came from and, in it, we will interpret this |
|
| 90:53 | . Now, one thing I should is that 55 turned into an A |
|
| 90:57 | , the 55 was AAA number we . When we assumed spherical, we |
|
| 91:02 | another number. If we assume another so that A has to be put |
|
| 91:07 | there for the geometry for sphere, would be 55. Um So I |
|
| 91:13 | said that the term is 55 for , 27 for cylinder. Now, |
|
| 91:20 | thing about this equation, which is bit odd note that the closure temperature |
|
| 91:26 | on both sides of this equation. we must not be done yet we |
|
| 91:30 | to solve for closure temperature, So you see a problem in solving |
|
| 91:35 | temperature on that equation. Uh What , what would we do next to |
|
| 91:41 | and solve that? Here's closure temperature this side. Super great. Here's |
|
| 91:47 | temperature in here problem though, Where's the problem? What? |
|
| 91:59 | we have to get on that But how are we gonna do |
|
| 92:01 | We have to, we got to it here. So we can, |
|
| 92:04 | we, we can get rid of log by putting it raised to the |
|
| 92:07 | , right? But then we have raise this to the power and we're |
|
| 92:10 | to the same problem. So what do you do? This is |
|
| 92:14 | example of where we can figure out easily, we solve this equation |
|
| 92:19 | So it's a very simple, what do is you, you have all |
|
| 92:23 | I'll explain in a minute where where we, we get all the |
|
| 92:27 | other terms, we get all the terms uh by uh experiment. But |
|
| 92:30 | we were to, if we imagine had all these other terms, the |
|
| 92:33 | and the R and the, and , the cooling rates and the |
|
| 92:36 | the D and the A, we all those things, we could substitute |
|
| 92:40 | some value for TT C here. it doesn't matter what you put in |
|
| 92:45 | . Let's just say, I always to pick 600 0 And by the |
|
| 92:49 | , I should point out these calculations to be done in Kelvin. You |
|
| 92:52 | substitute back and change it into but you put in a number 600 |
|
| 92:59 | say, and you do all your and then you get some number |
|
| 93:03 | It's probably not gonna be 600 to , let's say it's now 275 you |
|
| 93:09 | that 275 and now you do that you do the calculation and what you'll |
|
| 93:13 | is 270 put the 270 you get you put the 269 and you get |
|
| 93:22 | and you're done. So that's what's solving iteratively. When you have this |
|
| 93:25 | both sides, you just put it there until you get the same answer |
|
| 93:29 | both sides. You know, this an equation. Uh So long as |
|
| 93:32 | , it's that way. So we , that's not a problem. So |
|
| 93:40 | dealt with that little arithmetic issue, now consider the effects of these individual |
|
| 93:47 | on this closure temperature. We're getting trying to understand what closure temperature means |
|
| 93:51 | geo chronologic systems. Um activation energy this push, you have to get |
|
| 94:03 | get this thing going. What happens we increase activation energy? What is |
|
| 94:10 | ? Does the closure temperature get bigger smaller? Look at that equation and |
|
| 94:14 | me it gets bigger although it's Well, actually it's not what I |
|
| 94:21 | , explain why. OK. well, it's also, it's also |
|
| 94:29 | here though, but the good news it's in the numerator here. It's |
|
| 94:33 | the denominator of this denominator, which when that as that gets bigger, |
|
| 94:38 | gets bigger, which means this gets , which means it, it |
|
| 94:42 | I'm sorry, I'm not supposed to that. Um So when he gets |
|
| 94:48 | , um the whole thing gets what about what about cooling rate as |
|
| 94:53 | rate of cooling increases? What happens the closure temperature if this gets |
|
| 95:05 | Well, yeah, if this but it's even, it's, it's |
|
| 95:07 | complicated because if this gets bigger, this whole thing gets smaller, which |
|
| 95:14 | the whole thing, it's bigger. ? So the faster the cooling |
|
| 95:18 | the higher the closing temperature, um more important consideration might be dimension. |
|
| 95:25 | is something we've got a better feel . We don't have a good feel |
|
| 95:28 | cooling rage or activation energy. We a pretty good sense for the size |
|
| 95:33 | the crystal. That's little a here the crystal gets bigger or, or |
|
| 95:39 | I should, I should say, the diffusion domain gets bigger for the |
|
| 95:43 | , let's assume that the diffusion domain the same as the crystal as the |
|
| 95:49 | domain gets bigger as a gets What happens to the closure temperature? |
|
| 96:01 | . Don't look at the equation. do you think should happen as the |
|
| 96:05 | as the size of the crystal gets ? Is the closure temperature of the |
|
| 96:09 | gonna get bigger or smaller? What's smaller? Why close your temperature is |
|
| 96:17 | temperature at which you have to? is. OK. Suppose we had |
|
| 96:29 | tiny little crystal, you know, talking about having to diffuse outside of |
|
| 96:34 | . If, if, if, we don't have to, if we |
|
| 96:36 | have to go very far, then don't have to turn the heat very |
|
| 96:39 | to eventually get to the edge, . But if it's this big and |
|
| 96:44 | at the same temperature, it's gonna a lot longer to eventually find our |
|
| 96:48 | out. But if we turn the up, you know, it'll go |
|
| 96:52 | just as fast. So, as , as the crust, as the |
|
| 96:54 | gets bigger, I mean, the size gets bigger, we should |
|
| 96:58 | it to be more difficult to get at the same temperature. Excuse |
|
| 97:05 | a little bit like that. the bigger your house is, the |
|
| 97:08 | , the more it is to stay and warm. Um And so that |
|
| 97:12 | works out the same as, as gets bigger. This is the, |
|
| 97:16 | is a part of this quotient, ? So as this gets bigger, |
|
| 97:20 | gets smaller, but that's a part this quotient which goes into here. |
|
| 97:23 | the whole thing gets bigger. So I think the most intuitive bit of |
|
| 97:28 | is the bigger crystals should be easier retain their daughter products. Mhm Which |
|
| 97:43 | ? Oh, well, we, can treat each one of these separately |
|
| 97:48 | sometimes we'll group them together and D I and I squared, as I'll |
|
| 97:52 | just coming up, sometimes it's hard tell the difference between see D not |
|
| 97:56 | not is the intrinsic diffusivity of the . But when we do an |
|
| 98:02 | we can measure how far, how diffusion was moved. But the problem |
|
| 98:09 | is that was that because we moved short distance very slowly or excuse |
|
| 98:15 | short distance quickly, I get this , a short distance slowly or a |
|
| 98:20 | distance fast, we can get the result. So, so a short |
|
| 98:28 | slowly will give you one way or long distance quickly can, can give |
|
| 98:34 | the same. So, so many we don't break out DNA because they're |
|
| 98:39 | to answer your question. OK. So we, so we need to |
|
| 98:44 | determine the diffusion parameters of E and not on A squared for each |
|
| 98:51 | Um So I'm gonna describe how we these experiments and then we'll be done |
|
| 98:55 | this business. If you want to the data to, to figure this |
|
| 99:01 | out, you're gonna do an experiment the laboratory. Um Couple things we'd |
|
| 99:06 | to do. We'd like to make that our phase remains stable throughout the |
|
| 99:15 | . We're gonna be heating this thing to have diffusion happen, but we |
|
| 99:19 | want to heat it up so far it melts. For example, that |
|
| 99:23 | be, we would be measuring diffusion some other thing. Uh We, |
|
| 99:28 | even have to be more careful about than that. Suppose we're heating up |
|
| 99:31 | biotite, say biotite has water in . If you heat it enough, |
|
| 99:35 | water will go away and it's not anymore. So you gotta worry about |
|
| 99:40 | . Um You have to have some of how big your things are. |
|
| 99:44 | can start by measuring the size of crystals and assume that's the thing it'd |
|
| 99:48 | nice if the shape of the particles with one of our, our met |
|
| 99:52 | , our things, our diffusion But that's a small thing. Um |
|
| 99:59 | we'd like to heat simple heat it hot and keep it that way for |
|
| 100:03 | long time. So if we were do that, we can apply this |
|
| 100:09 | . Remember we have this equation where can measure diffusivity uh as a function |
|
| 100:14 | temperature. Well, this equation, we take the log of everything, |
|
| 100:19 | get log of Dion A squared equals of DNA squared plus this. And |
|
| 100:24 | ? That's why did we do that take the log? Because now we |
|
| 100:27 | an equation of a straight line where is Y, this is the |
|
| 100:33 | this is the slope and this is . So that's nice. Why is |
|
| 100:38 | nice? Because what we're gonna do we're gonna make, we're gonna do |
|
| 100:42 | experiment. And when we're done doing experiment, we can calculate by, |
|
| 100:47 | these approximations which came from those, long lists of equations I showed you |
|
| 100:53 | , we can calculate what the diffusivity or at least the diffusivity relative to |
|
| 101:01 | for a particular thing. And so everything is nice and straightforward, we |
|
| 101:05 | get a straight line on this And the slope of that diagram is |
|
| 101:12 | be the E over R. Remember , that's this, that's the |
|
| 101:20 | And the uh intercept up here is to be going to be this, |
|
| 101:26 | log of D on A squared. if we have, if, if |
|
| 101:30 | gives us E over R, the gives us D not on A |
|
| 101:36 | Now, we've got everything we need calculate the closure temperature. And here's |
|
| 101:41 | real data um from my lab a years ago where we measured diffusivity of |
|
| 101:47 | in calcite, drew a line there the E got the, we got |
|
| 101:51 | the stuff and we were able to a closer temperature of 60 degrees. |
|
| 101:56 | that's you could do an, you do an experiment, everything works out |
|
| 102:01 | . You get a straight line and we can say the closure temperature of |
|
| 102:04 | system helium in calcite is a number we can do that for all of |
|
| 102:09 | systems. And I won't go through anymore right now. Um Let's |
|
| 102:18 | we'll talk about that when we get South bars. Um Yes, like |
|
| 102:24 | missing a slide, but I'll just so we can do this, we |
|
| 102:27 | do this appro we can do this this, this calculation for all of |
|
| 102:31 | systems. And then we can, , and then we know which of |
|
| 102:35 | ones are sensitive to temperature and which more robust. Uh And I've got |
|
| 102:39 | , I've got a list of them on which I'll show you later. |
|
| 102:42 | some of them are very sensitive to . Some like this one have a |
|
| 102:46 | temperature of only 70 degrees, which that once we get up a little |
|
| 102:51 | above 70 degrees, we're gonna start our diffusion. It's gonna reset the |
|
| 102:56 | . Others might have a closure temperature 800 degrees. This is very important |
|
| 103:02 | know because when we date a you know, one is gonna tell |
|
| 103:07 | when it was crystallized that moment, crystallized at 800 degrees. The other |
|
| 103:11 | is gonna tell us when it would at some very low temperature. As |
|
| 103:16 | as we know what, what it . This is great news because one |
|
| 103:20 | us something about, you know, and something tells the other thing, |
|
| 103:24 | we have to be able to work out and it, it would be |
|
| 103:27 | if, if you had a pile your personal papers and one of them |
|
| 103:31 | , you know, this is when were born and this is when you |
|
| 103:33 | from high school and this is when got married and this is when you |
|
| 103:36 | that car and somebody might look and , well, well, these, |
|
| 103:40 | are, these are these pieces of all have different ages on how |
|
| 103:44 | how can we interpret that? you just know that, you |
|
| 103:47 | we don't, we don't, we born and we don't graduate high school |
|
| 103:51 | we don't buy a car all on same day. We have to appreciate |
|
| 103:55 | these documents mean. And that's the thing. We have to do is |
|
| 103:58 | have to appreciate when we date a by uranium, we get one thing |
|
| 104:03 | we date the same zircon by helium , we get a very different |
|
| 104:07 | So that's the concept that, that need to have in mind. I'll |
|
| 104:10 | you the actual numbers of each system a little bit. But this is |
|
| 104:14 | we go about figuring that out. been figured out for each system |
|
| 104:19 | So that's where that goes. People me, 000 That remember that our |
|
| 104:31 | temperature has a cooling rate in That's something we have to. |
|
| 104:37 | that, that's I'm glad you pointed out. Yeah. So when we |
|
| 104:41 | that, we, we can we can, we can get this |
|
| 104:44 | . This is just a constant, is a constant, this is the |
|
| 104:48 | . Again, this is what we're this. We measure experimentally, this |
|
| 104:52 | we measure experimentally this, we have assume we have to say, |
|
| 104:56 | given a particular cooling rate. And speaking, when we measure, when |
|
| 105:01 | want to calculate closure temperature, we say, let's assume a cooling rate |
|
| 105:05 | 10 degrees C per million years. As we saw, if you, |
|
| 105:10 | you choose a higher rate of you'll get a higher closure temperature. |
|
| 105:14 | when somebody says the closure temperature of and Y, they're usually assuming AAA |
|
| 105:20 | rate of 10 degrees C per But that's a ne no, you |
|
| 105:24 | , you can pop, you can , if, if you're in a |
|
| 105:26 | situation in which that's inappropriate you can in another cooling. But without, |
|
| 105:32 | specifying the particular geologic situation, people to calculate a number. So they |
|
| 105:37 | in 10 degrees C per million. All right, that was another hour |
|
| 105:43 | talking, maybe worth trouble. Um , um, so we're gonna now |
|
| 105:57 | , hey, we're gonna, we're go into our, our next, |
|
| 106:01 | first actual example of a system how works and what we can use it |
|
| 106:07 | . Um But why don't we take 10 minute break and we'll start again |
|
| 106:14 | with rubidium strontium dating, which is one of the uh uh things that |
|
| 106:19 | posted to uh canvas. All So first system we're gonna talk about |
|
| 106:30 | detail is the radium strontium dating In this, in this case, |
|
| 106:36 | have Verdi 87 is radioactive and decays strontium 87 by beta minus decay. |
|
| 106:43 | , we're transforming uh a neutron into proton, which is why we move |
|
| 106:48 | 37 to 38 on that equation. was one of the earliest methods used |
|
| 106:55 | geo chronology. It's not used as as it used to. It's superseded |
|
| 107:00 | some other methods, but it's an method to start with because it illustrates |
|
| 107:04 | well. Uh an important concept called Isac method which were, which is |
|
| 107:10 | of the way in which many systems around this problem of not knowing how |
|
| 107:15 | daughters we had to begin with. it's similar to other systems that |
|
| 107:20 | we'll talk about in the future. talk about potassium argon and some of |
|
| 107:24 | others. Um So learning about this , whatever everything we learn about ISOS |
|
| 107:29 | be carried over. So we're gonna this with every system we talk |
|
| 107:34 | We'll talk about the, uh, geochemistry of the parents and daughters. |
|
| 107:40 | Rubidium is a group one, a with an ionic charge of one and |
|
| 107:44 | ionic radius of 1.5. Um Strontium a charge of uh plus two and |
|
| 107:53 | very much smaller radius. Um so they're different from each other. |
|
| 108:02 | rubidium is a lot like potassium and is a lot like calcium. See |
|
| 108:08 | , these numbers here. And so you can find a mineral that has |
|
| 108:15 | in it or should I say potassium it and no calcium in it, |
|
| 108:23 | gonna start out with something that has rabid bearing mineral. Um A rabi |
|
| 108:28 | mineral will unlikely to crystallize strontium It's just not, it's not in |
|
| 108:34 | cards. So that's a problem because we don't know how many do we |
|
| 108:40 | with, then it's gonna make it to measure the age. Excuse |
|
| 108:50 | So I'll get to that question in minute, but let's just get a |
|
| 108:52 | more details about the system involved There's the equation for decay again, |
|
| 108:58 | uh the decay constant for bum 87 1.42 times 10 minus 11 per |
|
| 109:04 | Which if we flip that around and the closure temperature, we get this |
|
| 109:08 | temperature of 49 billion years. It like a very long time. But |
|
| 109:16 | that if we can find minerals that a large amount of rubidium in |
|
| 109:22 | then geologic time scales are appropriate to up enough stuff that we can measure |
|
| 109:29 | all of our cases. What we have to worry about is can we |
|
| 109:34 | enough data product to the mass spectrometer measure? And we can get around |
|
| 109:41 | problem in one of three ways, can make the sample have uh be |
|
| 109:47 | old. That's what we did with samples from the moon. If the |
|
| 109:51 | is very old, then we just long enough. We can, we |
|
| 109:54 | build up some of this daughter Even if decays very slowly, we |
|
| 109:59 | also have the sample um have a of parent in it. If you |
|
| 110:04 | , if you have like a potassium bar, then the decay of potassium |
|
| 110:08 | be easy to measure because potassium is the name, right. A third |
|
| 110:13 | you can fix this problem is even you don't have a lot of parent |
|
| 110:18 | even if the sample isn't super, old, we can get around this |
|
| 110:22 | . If we just have a really sample because we just shovel the sample |
|
| 110:27 | the mass spectrometer and then we can whatever got product is there. So |
|
| 110:32 | long as we can fix one of things, we'll be OK. And |
|
| 110:35 | why generally we, we find minerals have a reasonable concentration of radium. |
|
| 110:41 | , and the good news is Concentration doesn't have to be lots, |
|
| 110:45 | can have like 200 parts per 304 100 parts per million is uh |
|
| 110:52 | enough to date most geologic minerals because , because of our equipment is so |
|
| 110:59 | , we can measure teeny weeny little . So there are two isotopes of |
|
| 111:06 | , rubidium 85 and rubidium 87 rubidium is the one we're worried about because |
|
| 111:10 | radioactive. The ratio of the two today has a constant value. Uh |
|
| 111:17 | are four isotopes of stature, they're stable isotopes. They don't have a |
|
| 111:23 | life, they're all stable. Um the one in italics, there is |
|
| 111:29 | , as I mentioned before, that's one that is decaying that has been |
|
| 111:34 | is the product of the decay of 87. So we can say that |
|
| 111:39 | ratio of 86 to 88 is a number or the ratio of 84 to |
|
| 111:43 | is the constant number. But any that we use 87 will be always |
|
| 111:49 | . And that's why we say the of these minerals of these of isotopes |
|
| 111:54 | only approximate because it depends on what of material you're looking at. All |
|
| 112:00 | . So we can take our age and substitute in the actual parents and |
|
| 112:04 | this time. But, and so this case, we're using the, |
|
| 112:12 | , the, the subscript I for earlier, we used zero, but |
|
| 112:16 | the same idea. So the, amount of strontium we have is equal |
|
| 112:20 | the amount of strontium. 87. started with times the, the, |
|
| 112:24 | product of the amount of rubidium we 87 we have times ZT I |
|
| 112:30 | But here's our problem. It's very for the branch of 87 initial value |
|
| 112:37 | be equal to zero. So we a way to sort that out and |
|
| 112:43 | way we're gonna do this might seem bit odd at first, but |
|
| 112:45 | it works. First thing we're gonna is choose a non radiogenic isotope of |
|
| 112:51 | daughter as some normalizing factor. And going to pick in this case strong |
|
| 112:58 | 86 we're gonna divide both sides of equation by staunch of 86. So |
|
| 113:05 | we have a ratio of 87 to equals the ratio of 87 to |
|
| 113:10 | We started with times the ratio of rubidium distraction 86 times the ent and |
|
| 113:18 | looking at this and you're thinking, , how does that help us at |
|
| 113:21 | ? Because we've just moved from needing know the amount of staunch in 87 |
|
| 113:25 | we started with to the ratio of to 86. We started with, |
|
| 113:29 | still a thing we started with. the thing that was existing millions of |
|
| 113:33 | ago. How does this help? me? All right. The reason |
|
| 113:39 | helps is that we can take advantage the fact that when minerals are formed |
|
| 113:46 | an igneous rock. Anyway, we that these minerals have a different affinity |
|
| 113:54 | radium and strontium. So, so a natural segregation of rubidium strontium with |
|
| 113:58 | going into sub minerals a lot and others. And strontium going into some |
|
| 114:03 | a lot but not in some And so what that means is there's |
|
| 114:08 | be in a, in a rock simple same history. We're gonna have |
|
| 114:13 | dispersion of rubidium strontium ratios in those . Um And because no process fractionates |
|
| 114:22 | isotopes, the minerals that form with rubidium strontium ratios will form with exactly |
|
| 114:30 | same strontium isotopic values just after So if that doesn't make any |
|
| 114:38 | let's look at it graphically. Let's a granite site that has these minerals |
|
| 114:43 | it, appetite, pla glaze Kellar and muscular. At the moment |
|
| 114:49 | crystallization. These things would plot on graph like this in which the X |
|
| 114:55 | is is the parent divided by the daughter 87 rabid and divided by 86 |
|
| 115:04 | . And on the y axis, have the isotopic value abstraction. These |
|
| 115:12 | care about whether we're, they're incorporating Restrain. Some like Robin better, |
|
| 115:19 | like straum better. And that's why flock along the line here, but |
|
| 115:23 | don't care about that extra neutron in rabbin 87 versus shoot me the strontium |
|
| 115:30 | versus Stron 86. That's not a that is made. So the isotopic |
|
| 115:37 | of strontium in these minerals will start to be exactly the same because that's |
|
| 115:41 | was in the magma they crystallize into thing. That's the same, this |
|
| 115:45 | different. So now we know that 87 is radioactive, right? And |
|
| 115:54 | gonna decay, we know Rania makes . So over time, how are |
|
| 116:03 | point's going to evolve? What's gonna to this muscovite point? Say after |
|
| 116:13 | time, what is the radium 87 strontium? 86 ratio gonna do? |
|
| 116:18 | gonna go up, go down or the same. Excuse me? |
|
| 116:31 | that's too. I mean, half over time will it go up, |
|
| 116:34 | down or be the same? It go down? This ratio will go |
|
| 116:39 | , right? Why? Because this decaying and this is excuse me on |
|
| 116:47 | to do that because this is decaying this isn't right. So at, |
|
| 116:54 | some time equals zero, they're all same. But, but, but |
|
| 116:58 | on there, this is gonna be and this isn't. So the the |
|
| 117:03 | of these things of, of the axis is gonna go down for all |
|
| 117:06 | these things. What is the value the Y axis gonna do? What |
|
| 117:12 | Rubidium str what is Rabii 87 decaying 87? Right? So what's |
|
| 117:23 | what's the value of the Y ax gonna do gonna go up? |
|
| 117:28 | So we can excuse me over the medium 87 decays to strong |
|
| 117:36 | And so these things are gonna move way. Why have I drawn some |
|
| 117:40 | those arrows long and some of them , they're all moving in the same |
|
| 117:46 | . They're moving that way because they're down in the x axis, they're |
|
| 117:49 | up in the Y axis. They'll this sort of northwest trajectory on this |
|
| 117:54 | . But why have I shown that of these lines are longer than the |
|
| 118:03 | ? Not the rate the amount has this things, things out here |
|
| 118:10 | have a greater amount of rein. remember we said we go back to |
|
| 118:15 | flipping of the coins, we flip flip in this room. We only |
|
| 118:19 | four heads, we flip at the game, we get 3000 heads, |
|
| 118:23 | flip at the baseball game, we 20,000 heads. This is the baseball |
|
| 118:29 | , right? We're gonna have more in this situation because we started with |
|
| 118:34 | stuff this decay over here hardly even in the diagram because we had |
|
| 118:39 | we have, we start with We'll get zero. If we start |
|
| 118:42 | a GOB, we'll end up well, less than that because each |
|
| 118:46 | of these goes smaller and we go this way. So the proportion is |
|
| 118:51 | amount of decay is proportional to how we started out on this, on |
|
| 118:55 | edge. And so at some time , one, these values should have |
|
| 119:00 | , should have all migrated to that . And so then we can slap |
|
| 119:05 | line on there. And that line gonna be for the slope of that |
|
| 119:11 | is gonna be proportional to the We started out zero age, we're |
|
| 119:16 | , right? And over time, rotate up here, just rotate |
|
| 119:21 | the steeper. That line gets the the system is. And notice that |
|
| 119:27 | we do this way we get the , the intercept of this, of |
|
| 119:31 | line is the initial value that problem had to begin with. Well, |
|
| 119:34 | are we gonna do this if we know the initial value, this tells |
|
| 119:38 | the initial value, although we can of skip over the initial value if |
|
| 119:42 | want because we're really doing this just figure out the age. But now |
|
| 119:46 | got the age. This is called Isochron. This line, this blue |
|
| 119:51 | here, it is called an ISO it's line of equal age. And |
|
| 119:56 | is how we get around. The of not knowing how much daughters |
|
| 120:03 | Now. Clearly it works fine. . It's great. The problem is |
|
| 120:07 | got to have, you gotta make line, you gotta have three |
|
| 120:11 | let's say, from the same rock you can assume started out at the |
|
| 120:15 | and now have rotated up. So can't just do an iso on a |
|
| 120:19 | point. But if you've got some that has a suite of minerals that |
|
| 120:24 | happy saying have this, this, history, then the is Aron |
|
| 120:30 | The ISO method gets us around this of, oh my gosh, how |
|
| 120:34 | did we start with? Well, we go. We got it. |
|
| 120:39 | Here's an actual example of some rocks Texas. Um These are central |
|
| 120:46 | We'll, we'll use this, this central Texas rocks as an example because |
|
| 120:49 | why not? Um And we'll show for some other systems later on. |
|
| 120:56 | so here's, here's a uh uh real data from a, a rock |
|
| 121:00 | central Texas. This is, excuse , this uh this down here looks |
|
| 121:05 | one point, but it's actually the K Fels bar and wr means |
|
| 121:10 | rock. And because this is a granite with a lot of K |
|
| 121:15 | in it, the whole rock and K feldspar aren't very far apart from |
|
| 121:18 | other. So you got two points there. Then you got the Muscovite |
|
| 121:22 | and the bis head here, you a line through those points. This |
|
| 121:25 | , you know, these are the you measured the lab draw a line |
|
| 121:28 | that point. The slope of that is proportional to an age of |
|
| 121:34 | This granite from central Texas has got , you know, it's got a |
|
| 121:38 | Protozoic gauge of 1081 plus or minus . Based on the uncertainty of that |
|
| 121:43 | , it also tells us the intercept 0.8. That's interesting sometimes. But |
|
| 121:48 | the, from the point of view the age, it's not essential to |
|
| 121:52 | . So there you have it Central 1081. Um Here's an example uh |
|
| 122:01 | the moon and we get the same of story. Let me, let |
|
| 122:05 | point out that this rocks a billion old and notice the dispersion on the |
|
| 122:10 | of strong ratios go from essentially 0 900 huge range here. And that's |
|
| 122:17 | because we get a nice line. and we get, we get |
|
| 122:21 | a leverage on that line. We , we can do it fine. |
|
| 122:25 | we're gonna look at a rock from moon and of course, um this |
|
| 122:29 | from a, a rock called a . Anybody remember what a dunite is |
|
| 122:33 | your igneous class. Dunite is a that's 90% olive. So this is |
|
| 122:40 | olives and, and you might well how much rubidium or potassium is |
|
| 122:46 | an olive? Not very much, look at because you, and you |
|
| 122:50 | see that because look at the look how look how, how much |
|
| 122:54 | spread is that previous diagram went from to 900 0 to 900. This |
|
| 123:00 | from zero to 0.2. So there's little variation in the rubidium here. |
|
| 123:06 | if you let it sit around for billion years, you get a nice |
|
| 123:10 | that's defined here and we could still the same thing. And so here |
|
| 123:13 | get a bunch of a very low material but because it's on the |
|
| 123:18 | it sit around for a long They, they plotted it up, |
|
| 123:22 | got an age, the moon is billion years old. We knew |
|
| 123:27 | So, I mean, that, how we know we got, we |
|
| 123:29 | ages from the moon. Um So basically the Isochron method, couple of |
|
| 123:38 | we have to make here is of , that um we've had a closed |
|
| 123:42 | that, that nothing's been moving around the rock hasn't been altered and hasn't |
|
| 123:46 | reheated. Um What if we get uh a situation where the things |
|
| 123:53 | don't uh line up on a Um Here's an example um from a |
|
| 123:59 | in Nepal or those, those are data that were measured, not a |
|
| 124:05 | good line. Uh You can draw line on there sort of and say |
|
| 124:11 | about, that's about 20 million but it's a, it's a rotten |
|
| 124:16 | . The way this granite has been is that this was a, a |
|
| 124:21 | that was melting, it was formed the melting of previous sedimentary rocks. |
|
| 124:26 | those previous sedimentary rocks had a wide of material in them and they were |
|
| 124:32 | and they came to be found in granite, but this granite didn't mix |
|
| 124:36 | . And so this assumption we had the beginning that all of the strontium |
|
| 124:40 | in a crystal would crystallized with the value. Go back to remember |
|
| 124:47 | If we, as we can assume everything is fine. But what if |
|
| 124:51 | didn't start? What if this didn't out as a nice flat line? |
|
| 124:54 | if these were all over the place they didn't really come from, we |
|
| 124:58 | it the man is Lou granite. you could walk through it and it's |
|
| 125:00 | granite, but the, the rock was melted to produce that granite was |
|
| 125:05 | homogeneous. And so we ended up data that look like that crummy |
|
| 125:13 | So just because you can do, doesn't mean it works every time. |
|
| 125:18 | Then there's the second problem of how , what happens if we heat the |
|
| 125:22 | up? Um If you're, if are, for example, trying to |
|
| 125:29 | remember that first example, we showed how are we going to figure out |
|
| 125:32 | age of this paleontological interesting boundary? , we've got a granite that intrudes |
|
| 125:38 | . What's dated by the Rubidium Stron ? OK. That's a thought |
|
| 125:42 | then we'd have, we'd have an limit. On the age of that |
|
| 125:47 | thing. However, we have to and, and, and we'll go |
|
| 125:51 | this in all of our techniques. could go wrong? Well, what |
|
| 125:57 | go wrong here is a metamorphism. the heating of a rock is sufficiently |
|
| 126:01 | or high temperature, the strontium isotopes homogenize in the minerals in the |
|
| 126:06 | What does that mean? So, we said that we could take a |
|
| 126:12 | , let it sit for a T one would be the age of |
|
| 126:15 | rock. Let's take this rock and it. And in that case, |
|
| 126:20 | happens is all of the strontium isotopes equal as they recrystallize. And so |
|
| 126:28 | metamorphic times would be now re re uh establish a flat slope here. |
|
| 126:36 | we then let that evolve over time sets it to zero. We let |
|
| 126:41 | go over time and we could get thing here. But that wouldn't be |
|
| 126:45 | original age of the rock. That be the metamorphic age of the |
|
| 126:49 | which is fine if you're trying to out metamorphism. But if you were |
|
| 126:53 | in the original age of this rock out this wasn't the way to |
|
| 126:57 | Now, you may have been able figure that out already. If this |
|
| 127:00 | was metamorphosed enough to do this, could tell by the texture of the |
|
| 127:04 | . But if you're interested in this would tell you that if you're |
|
| 127:08 | in the age of the original rock you're interested in the cross cutting |
|
| 127:12 | blah, blah, blah. We've another technique, the uranium lead |
|
| 127:16 | which we'll talk about next, which do this. But this is just |
|
| 127:21 | show you what can go wrong with with that. And so to put |
|
| 127:27 | another way you could think of Iraq strontium, 8786 space versus time a |
|
| 127:34 | starts out, all of these minerals out with the same value that they |
|
| 127:38 | dispersing because they have different uh amounts them metamorphism brings them back all together |
|
| 127:44 | they go oxygen on their happy Second time. So we can measure |
|
| 127:49 | if we wanted to just some examples . Um There's a AAA nice |
|
| 127:55 | from Canada, got a nice bunch information in there. You see, |
|
| 127:59 | don't have a very big spread in things, but we still get a |
|
| 128:02 | age. Uh It's kind of kind uncertain value, but this was from |
|
| 128:06 | . We can do better than that . But that's that you would interpret |
|
| 128:10 | as the time in which these re of that grant of the nice took |
|
| 128:17 | . Um And here's just one more of metamorphism. I know that's not |
|
| 128:22 | your thing. But uh an interesting here of, of the scale of |
|
| 128:26 | re homogenization here are some examples from bunch of these uh metamorphic rocks in |
|
| 128:35 | . And if you plot the whole whole rocks uh from from this |
|
| 128:46 | just you take the whole rock and it up and measure the stranch in |
|
| 128:49 | that defines a line with an age about 548. But if you take |
|
| 128:57 | , the minerals from this one rock and you plot them glad you clays |
|
| 129:03 | biotite, it gives a different It gives a, a slope of |
|
| 129:07 | million here. So what we can here is that by looking at the |
|
| 129:12 | of, you know, tens of , the, the whole rocks define |
|
| 129:17 | original age of 540. But on scale of one rock, it has |
|
| 129:21 | re homogenized such that, that tells that they were metamorphosed 403 million years |
|
| 129:27 | . So when we re when we , it occurs on the scale of |
|
| 129:33 | , not kilometers. Um Let's Do I wanna talk about this? |
|
| 129:41 | period? Um OK, I will about maybe I have to talk about |
|
| 129:53 | . Yeah, I do have So the next thing I want to |
|
| 129:55 | about is um how we can use isotopic values of shales to tell us |
|
| 130:05 | about the provenance if you were interested , in uh an ancient shale. |
|
| 130:10 | just what's what's this broad information about what's being brought to us. You |
|
| 130:16 | measure this astron isotope value of the and tell you whether that, whether |
|
| 130:21 | a provenance was continental or more oce be, but to set that |
|
| 130:27 | I'm gonna talk a little bit about . Um, here's an example of |
|
| 130:35 | of the oldest bits of material we on the earth of, of |
|
| 130:39 | We've got a 4.4 billion year And what's interesting about this value is |
|
| 130:43 | gives this intercept, which is about lowest intercept that's ever been measured. |
|
| 130:50 | . It's, it's called Baby, stands for basaltic Achon. Best initial |
|
| 130:56 | a chondrites is a kind of And best initial says that this is |
|
| 131:00 | as low as anybody's ever measured. is how low the the the solar |
|
| 131:04 | started rocks that have a higher initial . Like let's go back to our |
|
| 131:11 | our granite from uh our, our granite from Texas that has a |
|
| 131:17 | of 0.8. Whereas we think the system started at a 0.69. So |
|
| 131:24 | is a lot bigger than that. that means is that the material that |
|
| 131:28 | melted to produce this granite was already part of the continent because in order |
|
| 131:36 | get a high value like that, have to have decayed. Rubidium. |
|
| 131:42 | is more likely to be in the than in the ocean. If |
|
| 131:45 | if you make a continent and let sit around for a while, the |
|
| 131:48 | value will go up. And so we measure uh values that are significantly |
|
| 131:55 | than these, these meteorites. We say that that's uh uh an expression |
|
| 132:01 | continent continent building. So, for , here's a granite from uh from |
|
| 132:08 | radium strontium value. It's got a of 0.71 that's modest. That's a |
|
| 132:15 | big value. And so again, means that a continent was, was |
|
| 132:20 | to produce that. Whereas if we to this grant, this uh basalt |
|
| 132:24 | Endy in Argentina, and we get same value. It's got an age |
|
| 132:29 | 472. That's interesting. But it's a value of 0.70 very low |
|
| 132:36 | That means that, that the uh mantle was involved when this was being |
|
| 132:41 | . So you were probably looking at rift zone that was bringing up mantle |
|
| 132:45 | than just recycling cotton. So you use that information to tell you a |
|
| 132:48 | bit about what was going on. so this leads to this business because |
|
| 132:54 | is more incompatible element than strontium in systems. When we look at the |
|
| 133:01 | , the rubidium strontium value of the is greater than the source it came |
|
| 133:06 | . So when we make, when , when we fractionate crust, we |
|
| 133:09 | continents rabid and moves from the mantle the crust. And so since rubidium |
|
| 133:17 | over time, this crust is gonna a higher 87 to 86 ratio as |
|
| 133:23 | rule values greater than 706 indicate extraction a crust. And this then has |
|
| 133:35 | used to try and figure out the the history of a continent like North |
|
| 133:40 | . See that green line there. , all these pink value, these |
|
| 133:45 | places here are granites that were formed the Mesozoic. And a bunch of |
|
| 133:51 | have done isotopic values of these things get their initial value. And to |
|
| 133:57 | west of that green line, we've values less than 706 to the |
|
| 134:02 | to the east of that line, have values higher than 706. And |
|
| 134:06 | that tells us that all of this here was added. It's not a |
|
| 134:10 | of sort of the ancient continent, was added in the Mesozoic, which |
|
| 134:13 | , which is the same thing that jobs tell me. So we've got |
|
| 134:18 | , a notion that continents have this value. Well, that then can |
|
| 134:23 | used for this provenance business that I that we're getting into because continents because |
|
| 134:29 | high 8786 value reflects old granitic We can look at the composition of |
|
| 134:37 | ocean and see how it changes the of the ocean is gonna be a |
|
| 134:43 | of what's being eroded into it. when we look at modern ocean, |
|
| 134:48 | can go select a piece of the Ocean water and the Atlantic Ocean water |
|
| 134:52 | so forth. It's very well The 8076 ratio in modern oceans doesn't |
|
| 134:59 | to change very much. And organisms take on this staunch of value |
|
| 135:05 | the water they live in. So can figure out what the strong and |
|
| 135:09 | 6 ratio of the ocean was in past by looking at shelves. And |
|
| 135:19 | can then use that variation to tell in some rocks. It's called strontium |
|
| 135:26 | stratigraphy. And it works when the 80 seventies situation of the ocean is |
|
| 135:31 | rapidly. And of course, that wasn't di meta modified by diogenes or |
|
| 135:39 | . And so here's how that works , here's a graph showing the 8786 |
|
| 135:45 | value of the ocean over time. And this is obtained by just |
|
| 135:53 | you know, getting, getting raky pods, anything you want, |
|
| 135:58 | measure them and you'll get the 8786 of the ocean in which those organisms |
|
| 136:05 | . And you can see it goes and down, up and down. |
|
| 136:08 | since about, let me just so just zoom on the, in on |
|
| 136:12 | last 100 million years since about 60 years old, since about 50 million |
|
| 136:20 | . It's a fairly monotonically increasing history . And so if you had some |
|
| 136:28 | that you didn't have good fossils and didn't have any other way, if |
|
| 136:33 | marine rocks, you might be able date them. This way. If |
|
| 136:36 | just pick a, you know, , measure the isotopic composition of, |
|
| 136:41 | your rock or a fossil in that ? Say it was 0.7085. |
|
| 136:47 | that means it's 15 million years Ok. Now, let's go back |
|
| 136:53 | this one. Why do you suppose goes up and down so much? |
|
| 136:58 | goes back and forth, back and . What, what is the, |
|
| 137:02 | is the reason for that change? could happen in the oceans or |
|
| 137:09 | You know, we say that the isotopic composition is a reflection of what's |
|
| 137:14 | added to the ocean. Why would , why would the uh staunch remember |
|
| 137:21 | ? 87 when, when that value high, when 8786 is high, |
|
| 137:26 | indicates continent and when it's low, indicates not continent or ocean. Why |
|
| 137:33 | it change like that so much glaciers you're, where you're holding the water |
|
| 137:40 | it's in ice or in the Well, that's a good place to |
|
| 137:44 | water. But I mean, it's , he said glaciers, um you're |
|
| 137:50 | the right track. We're trying to moderate the material that's going to the |
|
| 137:54 | , but really by not adding something the ocean by, by having glaciers |
|
| 138:01 | back water. We're not really worried the water, we're worried about |
|
| 138:05 | the, the, the sediment that's transferred to the water. So, |
|
| 138:09 | mean, you're on the right How are we gonna change things as |
|
| 138:12 | move into the ocean? You've, , you've been concentrating on the water |
|
| 138:16 | . But how can we can we as the water is delivered, |
|
| 138:21 | can we change its isotopic composition? isotopic composition of the ocean is reflected |
|
| 138:32 | what's dumped into it. And I mean the water but the stuff in |
|
| 138:37 | water. So why would the, like, for example, well, |
|
| 138:43 | just go to this one, for , here in the last 40 million |
|
| 138:46 | , we've increased a whole bunch What does that suggest has happened? |
|
| 138:54 | that we've had more continents being eroded in the past for the last 40 |
|
| 138:58 | years. The amount of old continent into the ocean seems to be going |
|
| 139:02 | and up. Can anybody think of , of a place since 40 million |
|
| 139:10 | ago? That's been eroding like Excuse me? What, what? |
|
| 139:24 | , I mean, not. I'm about really eroding where um I'm thinking |
|
| 139:34 | a mountain range not since for I mean, that's a good |
|
| 139:41 | That's old stuff, but there's a , there's a bigger mountain range that's |
|
| 139:44 | active than the Appalachians. Now, , we're talking, see, this |
|
| 139:47 | the thing that the app have. Appalachians really been evolving very much in |
|
| 139:51 | last 40 million years. Where's a ? That's where's in it? The |
|
| 139:57 | . That's, yeah, it's exactly . The Himalayas are the biggest mountain |
|
| 140:02 | . They happen to have a lot old trust in them. And the |
|
| 140:06 | between Indonesia really started going about 50 years ago. So here we see |
|
| 140:11 | the oceans, the presence of the , the Himalayas are an old and |
|
| 140:16 | range. They're gonna be dumping. know what, what river delivers more |
|
| 140:20 | to the oceans than any other Just to pass what river delivers more |
|
| 140:29 | . The Amazon. Yes. What delivers more sediment? Not number |
|
| 140:38 | Mississippi is a good one. But , the one in India, the |
|
| 140:44 | . Yeah, because you've got this mountain range right next to the |
|
| 140:49 | You know, the amount of sediment really a reflection of the relief of |
|
| 140:53 | mountain range. You got this huge mountain range right next to the |
|
| 140:56 | That's where more sediment is delivered. that's the, that is, that |
|
| 141:00 | uh reflected here we see in the of the oceans. The the the |
|
| 141:06 | of, of uh the structure, is a global figure. These |
|
| 141:14 | it doesn't matter. No. As said, if we said that earlier |
|
| 141:17 | , the uh the, the the 8786 ratio of is invariant in modern |
|
| 141:23 | go get us. And that's you know, they've, they've done |
|
| 141:25 | by scooping out some water from Hawaii some water from Calcutta and some water |
|
| 141:30 | the Aleutian Islands and they get the answer. So the, the the |
|
| 141:34 | oceans seem to be very well So when we see uh a graph |
|
| 141:40 | this, we can call on a source changing it all. And the |
|
| 141:44 | are a great example of how to that. They're the, they're the |
|
| 141:47 | active mountain range. They're really they're eroding like Tracy and they're dumping |
|
| 141:51 | bunch of sediment into the ocean that then reflected in the geochemistry of the |
|
| 141:56 | . And because, and so that this shows us is that if you |
|
| 142:00 | a sequence of rocks for which the aren't very good, you don't have |
|
| 142:03 | volcanic rocks, but it's a it's a marine sequence. You could |
|
| 142:08 | to, to measure strontium isotopes in sequence. Because from about 50 million |
|
| 142:14 | to the present, we have a uh correlation between the strontium isotopes of |
|
| 142:20 | water and the time obviously, if go back in time, it goes |
|
| 142:24 | and down because things like the Himalayas and start and go back and |
|
| 142:29 | What? So the Himalayas are an of how we're gonna make this ratio |
|
| 142:33 | up, right? We get a mountain range with some old continent, |
|
| 142:36 | it in there. Strontium isotope goes in the ocean. What would cause |
|
| 142:40 | to go down? And I don't just stop eroding if the 8786 ratio |
|
| 142:53 | really low. He said that that from the mantle, right? How |
|
| 142:58 | we more introduce more mantle into the ? Yes, you increase the, |
|
| 143:06 | increase the uh the rate of spreading the mid ocean ridges. We put |
|
| 143:10 | brand new basalt down there in the of the oceans. So when |
|
| 143:13 | when this ratio is going down, breaking up continents, we're making new |
|
| 143:18 | crust. So continents, continents make this value, go up, |
|
| 143:26 | , breaking open, make this value down. And that's why it goes |
|
| 143:30 | and down and up and down. are currently in a going up |
|
| 143:33 | And so we could use that for dating since about 40 million years. |
|
| 143:40 | would be a potential way to do . Um And it makes sense because |
|
| 143:44 | our understanding that continents have a high , oceans have a low value. |
|
| 143:57 | So, um from a, from practical point of view, I'd try |
|
| 144:02 | add this for most of our, systems. Um Modern mass spectrometer can |
|
| 144:09 | determine these values with a very small of material, very much less than |
|
| 144:14 | mg. So, in terms of practicality of dating these things, you |
|
| 144:20 | need a big sample. Uh If gonna do an iso you'd like to |
|
| 144:25 | a spread of veridian Stron values. But for rocks that are sort of |
|
| 144:31 | rocks, you know, it's, no problem dating rocks that are older |
|
| 144:36 | 20 I would say. Now I this slide many years ago, I'd |
|
| 144:40 | maybe you can even go down to 5 million years if you had the |
|
| 144:45 | conditions. So again, we've got three ways in which we can figure |
|
| 144:53 | out if the sample has a lot parent, if the sample is old |
|
| 144:56 | if the sample is big. if the sample is young, like |
|
| 145:00 | million years, you better hope you know, you've got a nice |
|
| 145:03 | hunk of sample. You're not gonna this by some little pebble or |
|
| 145:07 | Uh OK. Let's see. What is it? It's almost four. |
|
| 145:14 | me just take a moment. And I think you guys don't need to |
|
| 145:28 | that. So what I'm gonna do is move on to another topic unless |
|
| 145:35 | guys have any more questions about rum . Come here. Stop that. |
|
| 145:43 | you go. So we been as I said, is not a |
|
| 145:49 | commonly used technique these days because it, it does require three or |
|
| 145:54 | minerals from the same rock. it's good for an igneous rock. |
|
| 145:58 | can't date detrital minerals by bum strain you can't assume they have any sort |
|
| 146:03 | similar history. Uh But I introduce because it's the best way to describe |
|
| 146:09 | concept of an ISO which will be in other examples. So gonna close |
|
| 146:18 | we're gonna move on to the last of stuff that we've posted for you |
|
| 146:26 | . That's the Rubidium. Uh excuse , the uranium Thorium uh lead system |
|
| 146:34 | we'll start this now and we'll finish uh tomorrow morning. Ok. |
|
| 146:59 | System number two. 000, I , I didn't mention one thing. |
|
| 147:03 | just, I won't go back but say that. No, I'm not |
|
| 147:08 | bother them. Ok. System number , we're gonna talk about the decay |
|
| 147:13 | uranium to lead and thorium to Um This is OK in many |
|
| 147:21 | the gold standard of figuring out how things are if all you want to |
|
| 147:26 | is when this rock crystallized or when grain of sand crystallized, this is |
|
| 147:32 | number one, especially when we talk the minerals Zira uranium L Zircon dating |
|
| 147:40 | what a lot of people think geo , there's gobs of other geo chronology |
|
| 147:45 | . But if you had to, you had to pick one, this |
|
| 147:48 | what it is. And the reason because of the characteristic of lead and |
|
| 147:54 | , um the closure temperature of lead Zircon is a super high number. |
|
| 148:00 | when we date a Zircon, we're when it crystallized and what happened to |
|
| 148:05 | afterwards, if we cooled off slowly rapidly, if it was metamorphose doesn't |
|
| 148:11 | . And so that's why it's one the best ways to determine the crystallization |
|
| 148:14 | rocks. It's generally focused on accessory , minerals such as these Zircon monoxide |
|
| 148:22 | rio xeno spen. Because remember our ways we could have the, |
|
| 148:29 | the way that we're gonna work this is we're gonna pick minerals that have |
|
| 148:33 | rather high concentration of the rain. Zircon is one of those of those |
|
| 148:37 | . So we can't just pick nice . We've all heard of, you |
|
| 148:41 | , c spars and stuff like that there's no uranium in a F |
|
| 148:47 | but there's quite a bit of uranium a Zerka. Um So the geochemistry |
|
| 148:53 | these things, um uranium is a four charge and it has an ionic |
|
| 149:00 | of 1.08. Uh It's very similar thorium as it happens, it's also |
|
| 149:07 | similar to zirconium. Notice that zirconium plus four and has an ionic radius |
|
| 149:13 | . So when we're making a uh and zircon is the chemical formula |
|
| 149:19 | zircon is Z RSI +04. So we're making a zira and the magnets |
|
| 149:26 | , you know, we're picking stuff of the magma and zircon is |
|
| 149:30 | I mean zirconium is there. But a uranium comes along uranium will fit |
|
| 149:35 | into this spot, that was gonna for the zirconium. So substitution for |
|
| 149:39 | into the zirconium, easy peasy However, for the lead, the |
|
| 149:45 | has a charge of plus two and ionic radius of 1.4. It's different |
|
| 149:50 | both size and charge. And and, and remember when we're |
|
| 149:54 | remember back to your mineralogy or even geology, when we have an ionic |
|
| 150:00 | , we can worry about its the of things and the charge of |
|
| 150:04 | But what's the rule we have for when we're thinking about building a mineral |
|
| 150:08 | we, we, we tally up the charges, what all the charges |
|
| 150:11 | to add up to? Not at gotta add up to zero. |
|
| 150:18 | you gotta balance out to zero, ? You gotta have the negative charges |
|
| 150:21 | the positive, the negative charges come the oxygen and then you just gotta |
|
| 150:26 | that up to zero. And that's rule, there's no break in that |
|
| 150:29 | when you make something, it has be electrically neutral. So if you |
|
| 150:33 | gonna substitute a lead in for for a zirconium, you'd have a |
|
| 150:37 | problem and you, you might be to figure it out by substituting something |
|
| 150:41 | that, that balances it, but really difficult. Furthermore, this thing |
|
| 150:45 | really big, this, this thing really big compared to where it's going |
|
| 150:51 | . So this was what another reason uranium lead in zircon is so prized |
|
| 150:56 | because we have a very good feeling when we crystallize zircon, it will |
|
| 151:03 | uranium in it and it will not led. So that when we go |
|
| 151:09 | on to measure the uranium lead we can say that lead is from |
|
| 151:13 | situ decay. We don't really worry much about well, what, how |
|
| 151:17 | lead was there to begin with? just say not because of this, |
|
| 151:23 | of this geochemistry lead is very different zirconium. Uranium is very similar to |
|
| 151:29 | . So we can start with minerals have a lot of uranium in |
|
| 151:33 | which means that we don't have to forever for the game to came because |
|
| 151:36 | started out with relatively high values. and even out of crystal that the |
|
| 151:40 | , you know, zircon is a if it's a big guy, but |
|
| 151:46 | would be enough. Uh Because, know, because this substitution allows the |
|
| 151:50 | , excuse me, the uranium content be in a zircon might be half |
|
| 151:55 | percent, sometimes up to 1% uranium a mineral like that. And that |
|
| 152:00 | easy peasy to date it. Um , we've got another interesting situation because |
|
| 152:08 | got two isotopes of uranium. but we've got five isotopes uranium, |
|
| 152:12 | two of them have relatively long half . These other ones have really short |
|
| 152:17 | lives. But uranium 235 as we've , has a half life of 703 |
|
| 152:23 | years and uranium 238 has a half of about 4.5 billion years because uranium |
|
| 152:31 | has such a short, shorter uh constant um, or half life. |
|
| 152:40 | all the uranium 235 that ever existed earth is gone of the, |
|
| 152:44 | the abundance of, of uranium. of, of all the uranium left |
|
| 152:48 | the in earth is less than The ratio of uranium 238 to 235 |
|
| 152:53 | 100 and 37.88. And that's just reflection of the one decays faster than |
|
| 153:00 | other, but there's still some left not much, but that's, that's |
|
| 153:04 | big deal. Um So we can an equation for the decay of each |
|
| 153:10 | these uranium 238 decays to lead 206 eight helium particles plus six beta particles |
|
| 153:21 | some energy. I'll just, I'll this graphically in a minute. Uh |
|
| 153:26 | decay constant is there. If you that over and to calculate the half |
|
| 153:30 | there, it is again, 4.47 uranium 235 we can draw the same |
|
| 153:38 | . I mean, put it out again. In this case, we |
|
| 153:43 | uh lead 207 is our ultimate decay uh product. In this case, |
|
| 153:47 | get seven alpha particles and four beta . Now, oh and then there's |
|
| 153:55 | is another thing that, that decays lead. Thorium 232 is the only |
|
| 154:00 | lived isotope of thorium. So we have to worry about the other guy |
|
| 154:05 | we write the equation for thorium Dom decays to lead 208. And here |
|
| 154:13 | is, it has a half life 14 billion years. Now, we've |
|
| 154:22 | these isotopes. There's a bunch of of lead. The only ones we |
|
| 154:26 | to worry worry about are the ones red. You'll note that 206207 and |
|
| 154:33 | are in italics because they are They're the ones that decayed to |
|
| 154:38 | that, that, that decayed that produced by the decay of Uranium |
|
| 154:44 | It's, uh, it's kind it's easy to remember which decays, |
|
| 154:49 | if you just remember that uranium 238 the even one, there's 238 and |
|
| 154:56 | the even one decays to the even . So uranium 238 decays to lead |
|
| 155:01 | . The odd one decays to the one. Uranium 235 decays to lead |
|
| 155:07 | . And then you just gotta remember lead 208 is the bigger one that's |
|
| 155:12 | . Right. Now. You'll note this, this, this uh nomenclature |
|
| 155:18 | that those three are stable. There's isotope of lead which actually listed as |
|
| 155:24 | of half life, but notice how that half life is led to a |
|
| 155:29 | , has a half life of one 10 to the 17th years. That's |
|
| 155:34 | quite a long time. So it's is picking, you know, what's |
|
| 155:39 | difference between having a half life what is that? A billion, |
|
| 155:47 | ? That's 100 million billion years is half life. What's the difference between |
|
| 155:53 | and being stable? I know it's wanted to say, so somebody's measured |
|
| 155:58 | and says, well, if you 100 million billion years, you'd lose |
|
| 156:04 | of your le lead to a So from our perspective, we're gonna |
|
| 156:08 | that stable, which is nice because good to have one of your isotopes |
|
| 156:12 | be stable. So, here's an of the decay of uranium 238. |
|
| 156:18 | see uranium, the, the the red arrows are alpha decays. |
|
| 156:22 | blue arrows are beta decays and you out decaying and this goes on and |
|
| 156:30 | , no matter what you do, , you'll, you'll get down to |
|
| 156:33 | 206. Now you'll notice that some these things have two options. You |
|
| 156:38 | , like when you get to whatever is polonium, uh 100 whatever it |
|
| 156:45 | , 200 whatever it is, you see that some of them decay |
|
| 156:50 | this way and some of them decay this way and that's really just a |
|
| 156:53 | of ones that go one way or other. And that's the case for |
|
| 156:55 | lot of these, there's a, branch decay, it can go this |
|
| 156:58 | or that way. But the good is, is that you see all |
|
| 157:01 | branches that come back together to be to a six. So, uh |
|
| 157:07 | all we have to worry about So, uh and, and so |
|
| 157:11 | let's look at the individual decays a bit closer because we can, we |
|
| 157:15 | show that why it is. We make very much ignore these guys in |
|
| 157:19 | middle. Basically. Uranium 238 has half life of 4.5 billion years. |
|
| 157:24 | decays to thorium 234. Thor M has a half life of 24 |
|
| 157:31 | So we're moving right out of there quick indicates to proact tum 230 it |
|
| 157:36 | a half six hours. Then we back up to uranium. Uranium. |
|
| 157:41 | has a medium kind of half life 248,000 years for some things. That's |
|
| 157:47 | long half life. But for most what we're worried about, it's not |
|
| 157:50 | long. It decays down to thorium which has a half life of 75,000 |
|
| 157:56 | , which we will find there'll be situations where we're gonna worry about |
|
| 158:01 | but generally not. And then those decay radon 226 half life of six |
|
| 158:09 | years and then three days, three , 51 2nd, 35 milliseconds. |
|
| 158:16 | rest of these are all just teeny minutes and seconds, days, a |
|
| 158:20 | of days here and then all of comes eventually down. So there's only |
|
| 158:23 | couple of that long list that are than a few 1000 years. Um |
|
| 158:30 | basically we're gonna fit, we're gonna it down to lead 206 in about |
|
| 158:36 | times the age of the, of longest half life. So it takes |
|
| 158:42 | 300,000 years for this system be to completely in equilibrium. So if you |
|
| 158:51 | date uh a sample by uranium if it's less than 200,000 million |
|
| 158:56 | 200,000 years. Not really a good , but once you get above |
|
| 159:00 | this whole thing is in equilibrium and gonna ignore rocks that are less than |
|
| 159:04 | half a million years old. For , for this approach, we can |
|
| 159:09 | the same thing about the decay of 235 except it's a little less |
|
| 159:15 | Again, we have some, some decays and some beta decays and a |
|
| 159:20 | of branch decays that go one way another. Um But just as in |
|
| 159:25 | first case, uh it doesn't matter we'll all branch and it'll all end |
|
| 159:30 | in the same bucket down here. look at the uh half lives of |
|
| 159:35 | guys. Um, half life uranium 35 700 million years. But then |
|
| 159:45 | one's in our, this one's 3000, 2000 years, a couple |
|
| 159:51 | years, couple of days, minutes, seconds, seconds, uh |
|
| 159:58 | very short. And then we get here. So in this one, |
|
| 160:02 | even, what's the longest one in thing we got 32,000 years is the |
|
| 160:07 | one. Excuse me? Yeah. that's gonna, that's gonna obtain its |
|
| 160:14 | in 100,000 years easy. And then , we can look at the decay |
|
| 160:19 | thorium 232. It's even simpler. aren't as many possibilities. Only a |
|
| 160:27 | of branches and we get down there let 208. Uh Again, the |
|
| 160:33 | the decay rates and, and the things are pretty short. Here's a |
|
| 160:37 | of years and hours, days, , seconds, minutes, microseconds, |
|
| 160:43 | hours, microseconds. Ok. So just nothing. So that, that |
|
| 160:48 | is established really fast. And for our purposes, we can, |
|
| 160:53 | can just know that there are things the middle. Uh, but we're |
|
| 160:56 | gonna worry about how fast it but one thing we are gonna worry |
|
| 161:00 | in a minute later on, we to go back to or something later |
|
| 161:09 | like here. Yeah, we're gonna attention to the helium because strictly |
|
| 161:16 | that is a, that's a decay too. We can say uranium decays |
|
| 161:20 | lead, but just as well, decays to helium. And, |
|
| 161:26 | indeed, I'll tell this story more , I guess. Yeah, I |
|
| 161:33 | . But the very first rock that ever dated was dated by the uranium |
|
| 161:38 | method, uh, because they looked this equation and they said we got |
|
| 161:42 | helium. It's probably gonna be easier measure the helium, we'll measure the |
|
| 161:47 | . And uh they got, they an answer they weren't prepared for and |
|
| 161:51 | they measured the lead and they got different answer. So when we talk |
|
| 161:54 | uranium helium dating tomorrow in the it, well, just a bit |
|
| 161:58 | a spoiler. Let me, let , let me, let me ask |
|
| 162:02 | this consider cause your temperature, if were to measure in a mineral, |
|
| 162:10 | uranium lead age and the uranium helium . Both of these, the helium |
|
| 162:17 | helium uranium ratio should be proportional to right because this is all happening according |
|
| 162:24 | this half life business. So as goes on, you're gonna get more |
|
| 162:29 | more healing for every uranium that you get eight helium and that's just |
|
| 162:35 | build up over time just in just as in the case of when |
|
| 162:41 | have one uranium, it decays to leg and that's gonna build up over |
|
| 162:45 | . Now, if we were to at a single mineral and measure that |
|
| 162:52 | and look at the uranium lead ratio the uranium helium ratio in the same |
|
| 162:59 | might we expect those values to, give the same age or not? |
|
| 163:09 | the difference between lead and helium can you? Well, that's true. |
|
| 163:19 | , but then when, when they're of a, of a crystal |
|
| 163:22 | that gas versus solid thing is not important. There's this and there's |
|
| 163:37 | it's hugely 100 times bigger, 50 bigger. That means it's gonna be |
|
| 163:44 | to diffuse lead out of a crystal helium, the same crystal, whatever |
|
| 163:47 | that is, if you turn the up a little bit, you |
|
| 163:50 | we talked about how, you diffusion will start acting, it'll act |
|
| 163:54 | on something small, then something So when they date, when they |
|
| 164:01 | dated something they got, they well, let's do the helium because |
|
| 164:05 | get eight of them. That sounded a great, you know, that'd |
|
| 164:08 | easier. But it turns out there very many helium's in there. They |
|
| 164:13 | an answer that was much younger than were expecting because they didn't understand the |
|
| 164:18 | of closure, temperature. When you the rock up, the healing can |
|
| 164:22 | away. But we'll talk more about tomorrow. Um, ok. |
|
| 164:36 | all right. Um Here's just another way to consider the evolution of these |
|
| 164:42 | over time. Here is if we . Um let's see, let's |
|
| 164:50 | here's the proportion. So the solid represent the evolution of the parents starting |
|
| 165:00 | billion years ago. And the dotted represent the evolution of the daughters over |
|
| 165:06 | same period. So two ol uranium 235 is in lead is, |
|
| 165:11 | here in red. So you can that uranium 235 is dropping really fast |
|
| 165:18 | now has flattened out. We're almost of uranium 235 because we've gone through |
|
| 165:24 | six half lives of uranium 235 in space of the age of the |
|
| 165:31 | it's almost all gone. And that's we're gonna have all the uranium 27 |
|
| 165:35 | gonna get. But during that same period, uranium 238 in blue has |
|
| 165:41 | decaying and notice that it's, if started out at one, whatever that |
|
| 165:45 | , notice where we are now, basically at 0.5 because we've gone through |
|
| 165:51 | one half life of the, of 238 over the age of the |
|
| 165:57 | And if we look at this last here, uh the green, |
|
| 166:04 | the green one is an even flatter . We've gone at the age of |
|
| 166:09 | earth. We've gone down about 20% what we started with because it has |
|
| 166:14 | even longer half life. Ok. , well, th th this |
|
| 166:20 | this just shows us that we we can date really old rocks and |
|
| 166:25 | young rocks with these techniques because the lives are about right for our |
|
| 166:31 | And again, the favorite mineral for by this system and, and really |
|
| 166:39 | all systems. But like I when, when, when a lot |
|
| 166:44 | people think of geo chronology, this what they think, what they're talking |
|
| 166:47 | . If they don't have a more notion is dating zircon by the uranium |
|
| 166:53 | method. It's the most commonly dated . Uh because it, it starts |
|
| 166:58 | as I mentioned earlier with a high of uranium and a low concentration of |
|
| 167:03 | with lead. It's also very favored the closure temperature of lead is very |
|
| 167:10 | . It's so high that we don't a very good notion of what exactly |
|
| 167:13 | . It's above 750 degrees. It be 1000 degrees. Nobody really |
|
| 167:18 | It's just super, super hot, so high that it it withstands |
|
| 167:24 | you can metamorphose the rock and the lead will not leave the |
|
| 167:29 | which is great. If you want know the original age of some metamorphic |
|
| 167:33 | . Indeed, I skipped over The business we talked about what's, |
|
| 167:38 | do we know the age of the ? The oldest rock that we know |
|
| 167:42 | is this 4.1 billion year old rock Canada that's had its crystallization age 4 |
|
| 167:50 | , 4.1. It's a metamorphic but we're not talking about the age |
|
| 167:55 | metamorphism. We're talking about when it crystallized because Zircon has this high closure |
|
| 168:01 | . The other thing we like about is in the sedimentary environment. It's |
|
| 168:05 | tough, it's just as tough as corps. So when you're rolling around |
|
| 168:09 | and sandstone, they're staying there. as we'll talk about tomorrow, uh |
|
| 168:15 | dating is a very big deal these and zircons stay throughout the history. |
|
| 168:21 | so we can use dating of Zircons a provenance indicator because it stays |
|
| 168:27 | Uh Zircons come all the way down Mississippi River, you can date them |
|
| 168:32 | in uh you know, in New . Um they're still fine because even |
|
| 168:37 | they've traveled all the way from so they're, they're robust in the |
|
| 168:43 | environment, they're robust in the metamorphic , they're robust in the sedimentary |
|
| 168:48 | Um That's why they're great. And they have low uranium, uh high |
|
| 168:53 | and low lead. Ok. So could go about dating um uranium lead |
|
| 169:02 | by ISOS in the same way as described ISOS before we can do a |
|
| 169:08 | Aron for uranium 238 decaying just like we're going to normalize with a stable |
|
| 169:16 | of the daughter. So we're gonna by lead 204. We could do |
|
| 169:20 | and, and, and you can that and this technique is not used |
|
| 169:25 | lot and you might use it in to date some carbonate rocks. If |
|
| 169:29 | don't have a fossil in your, your limestone, some people have dated |
|
| 169:33 | uh limestones by this ISO method. same concept, these rocks, these |
|
| 169:39 | formed in an ocean, they had same um lead ratio to begin |
|
| 169:43 | They have a variation in uranium We get an iso can be |
|
| 169:48 | You could also do it with That's 238. You could do the |
|
| 169:51 | thing with uranium 235. Uh The is that the uh the decay. |
|
| 169:58 | you don't get the, the, abundance of your aim 235 is gonna |
|
| 170:01 | really low. Uh which makes it . You could do a nice |
|
| 170:06 | the thorium 232 as well. we are gonna take advantage of the |
|
| 170:14 | that in uranium lead tech uh we got two isotopes of one element |
|
| 170:20 | decay to two isotopes of another Both of the parents are uranium, |
|
| 170:25 | of the daughters are lead and this us. And, but, but |
|
| 170:34 | addition to there being two isotopes of , they have a different decay rate |
|
| 170:38 | different decay constant. So, we've that helps. So we're gonna use |
|
| 170:43 | fact that we've got 22 decay systems the same system, in the same |
|
| 170:49 | to check them against one another. , we could write the equations like |
|
| 170:58 | way, uh to uh to um their different uh decay constants here. |
|
| 171:05 | , I've written lambda eight to represent decay constant of 238 and Lambda five |
|
| 171:13 | the decay constant of 235. So are equations, we could write that |
|
| 171:18 | , that references the ratio of parents daughters to time. Um I |
|
| 171:26 | I think I briefly mentioned this before I think I didn't do it enough |
|
| 171:29 | we, when we see a star that on the, on the lead |
|
| 171:34 | , that means radiogenic lead. That we are, we are assuming that |
|
| 171:38 | have subtracted away whatever lead was there begin with. And in the |
|
| 171:42 | we can say that that was probably . So these two systems, although |
|
| 171:48 | have different uh decay constants might yet give you the same answer. So |
|
| 171:55 | you were to measure the lead 206 238 ratio in a in a sample |
|
| 172:00 | measure the 2072 35 ratio in the sample when you calculate an age from |
|
| 172:06 | , based on these equations, when get the same age, those that's |
|
| 172:11 | to have be a condition of they are concordant, these two |
|
| 172:17 | And so we could draw a graph these isotopic ratios. As described in |
|
| 172:24 | equations. We could draw a graph a line in which all of the |
|
| 172:28 | on that red line have the same in the two systems. So that |
|
| 172:36 | is called the Concordia line. Because , every point on there and you |
|
| 172:40 | see how some of them have have been annotated, there's the 500 |
|
| 172:43 | point, there's the 1000 million, the 1500 million and so forth. |
|
| 172:48 | some anything that lies on at that here, if you were to, |
|
| 172:53 | you were to fall down here to value and then calculate an age based |
|
| 172:57 | this 3.1 something that would give you age of 1500 million. If you |
|
| 173:03 | over here, it's a very different . It's not 3.1 it's 0.26. |
|
| 173:09 | because it's got a different decay you calculate in age, it would |
|
| 173:12 | give you 1500 million. So you've Concordia, all of this red line |
|
| 173:19 | called the Concordia line. That's good . Well, um see where we |
|
| 173:30 | , we'll get back to the Concordia in a minute. Um Going back |
|
| 173:37 | our two equations. Um we could divide these two equations by each other |
|
| 173:47 | rearrange them so that we could get relationship of the two isotopes of the |
|
| 173:56 | radiogenic isotopes L 207 and L That's uniquely dependent on just this thing |
|
| 174:04 | . The, the ratio of uranium to 238 which is a constant. |
|
| 174:10 | , that's just a constant. We what that value is. That's 100 |
|
| 174:12 | 37. And then there's this the, the, the, |
|
| 174:18 | the e, the land T for two different systems. Now, the |
|
| 174:22 | here is that although we can measure or excuse me, we can measure |
|
| 174:27 | lead isotopes in the lab. That's thing, we can go measure, |
|
| 174:32 | We can't solve this equation for T once again, T is in the |
|
| 174:37 | in two different places. So we solve for T. But what you |
|
| 174:42 | do is just plug in tea a of times and make a day to |
|
| 174:47 | . You could make a table like and say for various, I |
|
| 174:51 | and this is a very coarse table I've made up. It, it |
|
| 174:55 | every 400 million years. But you , you could make this table around |
|
| 175:01 | value you wanted to suppose. you measure the value in the lab |
|
| 175:05 | then you could just increment it very to figure out what it was. |
|
| 175:10 | what I'm saying is if you measure in the laboratory, you can then |
|
| 175:15 | , that's, that corresponds to 1.6 years. So this is one way |
|
| 175:19 | go. If you could just measure two isotopes that are the daughter |
|
| 175:25 | you didn't even have to measure the situation. Measure the lead because they |
|
| 175:30 | at different rates. The they, grow at different rates. The lead |
|
| 175:36 | to lead 206 ratio is a unique of the age. Uh So that's |
|
| 175:42 | way to go, but there's still power in this Concordia diagram. Um |
|
| 175:50 | let's just say, for example, have a point that we measured on |
|
| 175:53 | , on our graph. It's that dot We've got three different possibilities for |
|
| 176:00 | the age here. If we measure down, we get the uh the |
|
| 176:05 | 207 age. If we measure across the Y axis, we can get |
|
| 176:10 | lead 206 age or if we measure the in from the origin up to |
|
| 176:18 | , that's the lead 207206. um it is the case now that |
|
| 176:30 | is what, what's shown here in blue dot would be called a discordant |
|
| 176:34 | where the lead 207 age and the 206 age are not the same because |
|
| 176:39 | doesn't plot on that on that red . Nowadays, we can be very |
|
| 176:50 | about which zircons we look at and know just by visually inspecting them which |
|
| 176:55 | look nice and which ones don't. we can really try and avoid discordant |
|
| 177:03 | . But I'm gonna tell you about points because you may have to read |
|
| 177:07 | of something. You know, if go into some region where the geology |
|
| 177:10 | not been well done and some of geo chronology might be old, there'll |
|
| 177:15 | discordant data. And so what's what has done now and was done |
|
| 177:21 | the past is that we will get rock, whether it's a granite or |
|
| 177:26 | rite. These would be the most rots. Zircons are more common |
|
| 177:32 | in uh Fels pye rocks. So are mostly found in rites and |
|
| 177:42 | And so the, the, the from a Strat democratic point of |
|
| 177:46 | , a rite is best because it granites, it has zircons in |
|
| 177:51 | But even if you find a nice , you're gonna want to support the |
|
| 177:56 | segregate them. Here's a picture of zircons from a particular rock and you |
|
| 178:00 | they're, they're not all the they've got different sizes and shapes and |
|
| 178:05 | got pollutions and stuff in them. what will be done is after you've |
|
| 178:11 | the, after you've obtained these zircons , and by the way, so |
|
| 178:16 | would get a bunch of zirconi, gotta crush up the rock and then |
|
| 178:20 | got to separate the heavy minerals from light minerals using this this heavy |
|
| 178:25 | these liquids that have really high And so you can pour your powdered |
|
| 178:29 | in there and all your quarts in Fels bar will actually float on top |
|
| 178:33 | this liquid and your zircons will sink to the bar. And so eventually |
|
| 178:37 | get a nice concentrate of zircons and like that. But even then you |
|
| 178:42 | sort these zircons by these characteristics like size, their color, their shape |
|
| 178:47 | their magnetic susceptibility. The magnetic susceptibility nice because that's a machine you can |
|
| 178:53 | to do that to sort them by . You have to get a piece |
|
| 178:56 | , you get tweezers and actually move blue ones over here and white ones |
|
| 179:00 | here. It's very tedious, but it will show up a show a |
|
| 179:05 | on the diagram. But however, do it, you can separate these |
|
| 179:10 | out and then you will see the on this diagram. So this diagram |
|
| 179:19 | called the Uranium L Concordia diagram or called the weatherill diagram named after George |
|
| 179:24 | who thought it up. And if uh had discordant data and, |
|
| 179:35 | and this was very common in the times of doing this, let's say |
|
| 179:39 | 19 eighties, you'd see this sort data a lot, not so much |
|
| 179:43 | . But if you have uh discordant such as this, they, they |
|
| 179:48 | plot in a fashion such as And so the red line as I |
|
| 179:53 | is called the Concordia line. The line is called the Discord line. |
|
| 179:58 | it's, if, if you get nice straight line like that, |
|
| 180:01 | it's potentially interpretable. You've got an intercept and a lower intercept. Now |
|
| 180:10 | to interpret these things. There's two going on here. Um We've got |
|
| 180:18 | situation in which these, these points started up here and have been drawn |
|
| 180:23 | on this line based on, on unknown, some, some difficult to |
|
| 180:27 | sort out issues, but maybe because , of, of alteration, maybe |
|
| 180:34 | of diffusion, maybe because of, don't know but some, but it's |
|
| 180:38 | that sometimes these things will start up and be drawn down towards the |
|
| 180:43 | In which case, we interpret the intercept to represent the beginning where this |
|
| 180:47 | started. And these things have been away. And if you have a |
|
| 180:50 | line like that, OK, that's . Unfortunately, there's another way to |
|
| 180:56 | that is that maybe, uh and me go back to this picture of |
|
| 181:01 | . Uh And we see, we have a really good example here, |
|
| 181:07 | show you some others in a minute tomorrow, but like, all right |
|
| 181:14 | , see right there, there might a little bit inside that Zircon, |
|
| 181:17 | a little bitty round spot in Maybe there's one there, maybe. |
|
| 181:23 | I told you that Zircons are they'll stick through the igneous metamorphic or |
|
| 181:28 | environment. They are so robust that can take an old rock like an |
|
| 181:33 | granite or an old sandstone and you it under the right tectonic conditions, |
|
| 181:37 | could melt it. Ok. When melt that rock, you melt all |
|
| 181:42 | quarts, you melt all the F , you melt all the bow |
|
| 181:45 | easy peasy. The zircons still hang there. You can't even melt some |
|
| 181:51 | . And so that zircon will be called um xeno christic. It's from |
|
| 181:57 | older crystals, it's foreign to this magma and that little bit of old |
|
| 182:04 | can hang in there. And then new zircon as this magma then crystallizes |
|
| 182:09 | new zircon will form around that old . So you have a core of |
|
| 182:14 | and a younger bit. And so you were to analyze that entire zircon |
|
| 182:18 | some group of zircons, the age would get would be some combination of |
|
| 182:24 | age of the old bit and the of the younger stuff that grew around |
|
| 182:28 | . And so that's how one way you might interpret an an orientation like |
|
| 182:32 | is that we're mixing between a population old zircons and a population of young |
|
| 182:39 | . And that's, it's a mixing in here. In which case, |
|
| 182:42 | crystallization age of your rite would be here. OK. Um So Accordia |
|
| 182:52 | Cordia upper intercept. So here we the, the, the, |
|
| 182:56 | the conundrum, the upper intercept can interpreted as the age of crystallization or |
|
| 183:04 | age of the inherited component stuff that there and didn't melt. Alternatively, |
|
| 183:11 | lower intercept can be interpreted as the of crystallization or the time in which |
|
| 183:16 | lead loss event has occurred. How we gonna be able to tell the |
|
| 183:20 | between the two? Oh Well, we, uh uh let's, let's |
|
| 183:28 | about this l loss idea a little before we go on. Um So |
|
| 183:35 | is um some of these weather diagrams the top here, we'll ignore this |
|
| 183:41 | down to the bottom. Um Imagine rock that is say 1700 million years |
|
| 183:48 | . It gets to this point where red dot is, but then something |
|
| 183:52 | to it and it, it, zircons that had this condition are, |
|
| 183:59 | pulled down towards the, the, growth of new zircon or the loss |
|
| 184:05 | , of uh lead you need, pulled down this way and then |
|
| 184:13 | this disturbance event is over and they to evolve, moving from, from |
|
| 184:19 | place to these to this new place . So this, this, this |
|
| 184:26 | of data now looks like this. go from here through here, through |
|
| 184:31 | . And so what we would say that, well, this looks like |
|
| 184:35 | rock is this old, but it disturbed at this time. And so |
|
| 184:41 | would interpret this to be the real and this is just a reflection of |
|
| 184:45 | that happened to it. Um what happen to it? Variety of |
|
| 184:52 | Actually, I don't think I want trouble you with that. Um But |
|
| 184:56 | just take some more good examples of data. Here's, we're going back |
|
| 185:02 | central Texas again. Here's a similar and the one we looked at for |
|
| 185:07 | Stron. And here we have a of zircons. These are all zircons |
|
| 185:12 | we have a bunch of points which , and, and notice that we're |
|
| 185:16 | in here, we're not showing the the, the, the uh origin |
|
| 185:20 | this diagram. We've zoomed in to look at the ages from 800 to |
|
| 185:26 | . And we've got one point here plots pretty close to Concordia and then |
|
| 185:30 | other points that fall off here like and what these points will have, |
|
| 185:35 | points will have been segregated by, I said, by color or by |
|
| 185:40 | susceptibility or by whatever they chose. , but this is, there's a |
|
| 185:45 | from 1992 and back then, the spectrometer were usually not sensitive enough. |
|
| 185:54 | , I should say it depends on kind of mass spectrometer you have. |
|
| 185:58 | back then often you would be looking each one of these points represents more |
|
| 186:07 | one zircon. They had to push sample in there to measure the sensitivity |
|
| 186:12 | the machine. Usually couldn't handle just grain because they couldn't tell the signal |
|
| 186:17 | the noise. So they would use few grams, 10 or 20 or |
|
| 186:24 | . Um And so that's what each of these things would be and they |
|
| 186:28 | vary again by color or magnetic susceptibility size or shape. Somehow. They |
|
| 186:35 | wanted to segregate them and, and a spread on this diagram. Of |
|
| 186:40 | , if all the, if all points land on the red line that |
|
| 186:44 | all concordant, it doesn't matter how segregate them. But the point was |
|
| 186:47 | that they, they would expect this distribution of data. So if you |
|
| 186:55 | a nice line, then you can that up and see where it intercepts |
|
| 186:59 | . And that's what they're after. were expecting a line that was |
|
| 187:05 | But once you got data like you would draw a line 1082. |
|
| 187:09 | right, there's your answer. That's age of the rock. Notice that |
|
| 187:12 | the same age we got for the strontium age, which was what, |
|
| 187:16 | 81 or something. I can't It's the same. Um So I'm |
|
| 187:24 | skip that. Oh no, I'm . Well, I'm just gonna tell |
|
| 187:27 | that when we talk about zircons and melting, there's a, there's a |
|
| 187:34 | we can figure out whether a zircon gonna melt or not. It depends |
|
| 187:37 | the temperature and the composition, but zircons they don't melt. So we |
|
| 187:41 | have to worry about that. But is an example of where we could |
|
| 187:45 | a rock that uh has an inherited . This is a, this is |
|
| 187:51 | , a sand uh a granite from Arizona, from Arizona, excuse |
|
| 187:56 | near Tucson. And we see that a bunch of points here. There's |
|
| 188:01 | of them there, there's one of there and they, they define a |
|
| 188:04 | line that goes up here to 1400 and goes down here to 66 |
|
| 188:12 | So if we go back to that slide, we can interpret the upper |
|
| 188:19 | as the crystallization age or the lower as the crystallization. What are we |
|
| 188:26 | use to figure out what to Well, you, you might want |
|
| 188:34 | have a geologic map of the This is a granite, let's |
|
| 188:39 | right? This granite is intruding some older, younger rock or older |
|
| 188:43 | right? So you go to the and, and in this case, |
|
| 188:49 | granite is intruding uh Jurassic rocks. does that tell you about the age |
|
| 188:58 | the granite? It has to be than Jurassic, right? So the |
|
| 189:01 | crystallization age of this granite can't be . It could be this. It's |
|
| 189:07 | 66. So how do we interpret ? That this is a rock that |
|
| 189:13 | the older mesozoic rocks. It's a rock itself, it intruded 66 million |
|
| 189:18 | ago. Why does it have this behavior is because the rocks that melted |
|
| 189:26 | million years ago had some zircon in those zircons did not completely go away |
|
| 189:33 | that magma was formed. And so that magma is formed, and then |
|
| 189:37 | magma crystallizes new zircons start to grow on top of the old zircon. |
|
| 189:43 | this one, either this one has particularly well represented old bit. This |
|
| 189:48 | has a poorly represented old bit and just a mixing between the two. |
|
| 189:54 | so you have to combine your understanding how these zircons might be distributed on |
|
| 190:00 | diagram with your understanding of the local . So not hard to say this |
|
| 190:05 | was crystallized 66 million years ago because can't have crystallized 1400 million years ago |
|
| 190:12 | it's intruding rocks that are only 300 years old. OK. OK. |
|
| 190:22 | Yeah, that's what this, that's this says here. It's intersect |
|
| 190:25 | this no, this value because remember red line curves down, right? |
|
| 190:30 | so this will intersect at 1413. what that means. Yeah, I |
|
| 190:38 | , it may intersect at a really number. But I mean, because |
|
| 190:41 | , well, if we, if are modeling this as a straight |
|
| 190:45 | it will have to inter it, will have to intersect because the red |
|
| 190:49 | curves and curves only in that So yeah, um let me show |
|
| 190:57 | another example of inherited components of this from even older data. But the |
|
| 191:05 | idea is still the same. This from the Idaho Baffle. And here |
|
| 191:09 | have another pretty clear example of Here, we have four points which |
|
| 191:15 | cluster is really close to the, the uh Concordia line. And then |
|
| 191:19 | all zip off towards 1700. Um should point out, let me go |
|
| 191:25 | to this one. Not only do look at the geologic map to say |
|
| 191:30 | this rock intruded mesozoic rocks, but we look at the whole other region |
|
| 191:35 | what's the sort of, what's what's the basement rocks in this region |
|
| 191:39 | might have been melted to produce There's a bunch of rocks in Southern |
|
| 191:44 | that have an age of about So it's not surprising that the age |
|
| 191:50 | the inherited component is the same as we expect to find in the lower |
|
| 191:55 | in this region. And we see same thing happening here in uh in |
|
| 192:02 | , the broad age of the crust the deep, the pre the precambrian |
|
| 192:08 | . If you go and look at the precambrian rocks in Idaho S 1700 |
|
| 192:12 | a real common thing to find. so the fact that this thing seems |
|
| 192:16 | be mixing between 47 and 1700 is normal. That's the age of the |
|
| 192:25 | that melted. But the, all zircons didn't know. And so we |
|
| 192:32 | this as a mixing line more once , moreover, this, this Eocene |
|
| 192:39 | granite is clearly an Eocene granite because intruding rocks that are olden. This |
|
| 192:45 | not a precambrian rock because it's intruding stratum. Um It's hard to |
|
| 192:59 | I'll give you another example from something I published uh from my phd |
|
| 193:04 | many years ago. Uh And this a little bit of a complication of |
|
| 193:09 | . This is Zircons from a granite Mount Everest. And here we show |
|
| 193:16 | single zircon and by the way, , these, this, this, |
|
| 193:20 | example here from 1981 all of these are multiple zircons because again, the |
|
| 193:26 | spectrometer was not sensitive enough to be to measure a single grain. Uh |
|
| 193:31 | the best they could do. But this paper, which is just a |
|
| 193:35 | years later, but it was had more modern uh uh mass spectrometer. |
|
| 193:41 | of these points are singles or Now as we move it up in |
|
| 193:48 | , we'll be talking about measuring little of zircon. But here we've moved |
|
| 193:51 | the point where we can measure one . This was 25 sir. |
|
| 193:57 | but as we start measuring smaller and bits, we can start seeing more |
|
| 194:04 | . And here we have a granite we are interested in figuring out the |
|
| 194:07 | of. And so a bunch of were collected and they were all analyzed |
|
| 194:12 | you see that they spread out all the place here. Um But one |
|
| 194:16 | them plotted pretty much right here at million, but all of these other |
|
| 194:22 | plot all over the place. And you draw two lines between these four |
|
| 194:28 | that goes up to 500 million, one with a different slope still intersects |
|
| 194:34 | you, you know, because of curve there, but it intersects at |
|
| 194:38 | billion. And if you look at geology of the region, we know |
|
| 194:43 | this was a Miocene granite, that's a problem. But we also could |
|
| 194:49 | and see what kind of rocks are there that could have been melted. |
|
| 194:52 | are quite a lot of four division in this region. 500 million, |
|
| 194:57 | also precambrian rocks that are about 2 . So what we have is not |
|
| 195:02 | single source of mixing, but we the, the juvenile material down there |
|
| 195:07 | 20 then a range of, of zircons that might be between 502,000. |
|
| 195:12 | that we're just seeing the, the of that here. Um So this |
|
| 195:18 | the same idea we're looking at but now it's a more complicated inheritance |
|
| 195:23 | multiple sources. If you went back tried to do this sort of |
|
| 195:28 | say in the 19 seventies, they have done single grains, they would |
|
| 195:32 | done 10 or 15 grains at the . And they would have probably defined |
|
| 195:35 | sort of raggedy line that was somewhere the middle here, that would have |
|
| 195:39 | a state, they would have they would have probably said, |
|
| 195:42 | you know, that's a mixing line 21.6. And that probably that these |
|
| 195:50 | don't suggest a single mixing line because data are more sophisticated because now we're |
|
| 195:56 | at one grain at a time, I say nowadays, one grain at |
|
| 196:01 | time is sometimes too coarse. Uh we'll say, the next, the |
|
| 196:05 | step up in technology is shooting these with lasers. Um No, and |
|
| 196:14 | we are the uh what these data produced by a technique. Let me |
|
| 196:27 | . Where am I am I going this? Uh Oh I gotta do |
|
| 196:44 | at the end. Um OK, do that later. Uh These data |
|
| 196:57 | produced by a technique called thermal ionization spectrometer. Well, I'll, we'll |
|
| 197:03 | , we'll talk more about that But what's required of this technique is |
|
| 197:08 | the Zircon, putting it in some and dissolving it entirely and then measuring |
|
| 197:13 | bulk isotopes on that whole crystal and it shows up like that. But |
|
| 197:22 | since about this time, I mean , yeah, since about this |
|
| 197:30 | there have been other machines available. And uh as time goes on, |
|
| 197:37 | machines have gotten a lot cheaper. what they can do as shown |
|
| 197:42 | these little white circles, you can take a single zircon like this one |
|
| 197:49 | shoot it with a laser beam or ion beam and, and liberate material |
|
| 197:54 | that teeny little portion of the And then that that material is then |
|
| 197:59 | and then sucked up into a different of mass spectrometer, but measured, |
|
| 198:03 | the isotopes, same sort of And you can understand what the age |
|
| 198:07 | that part of the crystal is. me, that part of the |
|
| 198:11 | as opposed to that part of the . And you can see this, |
|
| 198:14 | grain seems to have a core so , that crack there and then there's |
|
| 198:19 | other bit around it. And nowadays can go in there and grab that |
|
| 198:25 | , that's 1991 and that bit there 203. So suppose this came, |
|
| 198:32 | , this is from a nice, this could just as easily have come |
|
| 198:35 | a, from a, a And if you were to date this |
|
| 198:41 | by the old method in which you the whole thing, you're not gonna |
|
| 198:45 | 1900 you're not gonna get 200 you're get what uh 1700 you know, |
|
| 198:50 | , some, some, some uh value. And if you're interested in |
|
| 199:00 | old history of this rock, you , you wanna know about the |
|
| 199:03 | if you're interested in the young history this rock, you wanna know about |
|
| 199:06 | 200 but doing the old way, get a 1700 which doesn't mean nothing |
|
| 199:11 | 1700 million years ago to this rock the, the interesting times are 219 |
|
| 199:17 | . But you wouldn't see that in old ways. Um And this just |
|
| 199:22 | another example of a, of a core in there. That's a little |
|
| 199:26 | bit, that might be quite a older. Uh And so we have |
|
| 199:31 | pay attention to that kind of Now, um Let's see. Um |
|
| 199:48 | , however, the Zircon's plot kind nice. Here's a bunch of Zircons |
|
| 199:52 | are all concordant. They're, they're all on top of one another, |
|
| 199:55 | they go, if you take the of all these guys, you can |
|
| 199:58 | a nice age for this volcanic rock 377. Um But sometimes you can |
|
| 200:07 | that there's clearly a history going on these zircons that are complicated. There's |
|
| 200:11 | metamorphic outside which is unzoned, an inside that is zoned. Uh And |
|
| 200:20 | need to be able to sort that . Here's another example. This is |
|
| 200:26 | a rite. This is another one my rocks that I dated to. |
|
| 200:29 | this was a, a rite that involved in a fold. We were |
|
| 200:33 | in the structural history of this So we wanted to figure out how |
|
| 200:36 | the fold was. We dated, youngest rock that was folded and this |
|
| 200:40 | how this was done in, I know, 2000 and when was this |
|
| 200:47 | 2010 maybe. And it was done at uh um and we have a |
|
| 200:53 | here that can take these zircons and little, you can see the little |
|
| 200:57 | in them. These little holes is what the zircon looked like. |
|
| 201:01 | these holes were added as we zapped zircon and then liberated the material from |
|
| 201:07 | and analyzed that bit. And you see that the ages are given in |
|
| 201:11 | here. And you can see the of these zircons, 100 microns. |
|
| 201:14 | these are pretty big zircons and these were pretty clean zircons. We |
|
| 201:19 | see any really serious cores in but we went ahead and zapped them |
|
| 201:23 | over the place because we didn't know weren't gonna be cores. The ages |
|
| 201:26 | all about the same. We plot on a histogram and we get an |
|
| 201:30 | here. So these ages are calculated with just the uranium 238 approach because |
|
| 201:39 | , they're often discordant when they're this . And so we take it, |
|
| 201:43 | , we assume that if we put a bunch of uranium 238 ages, |
|
| 201:51 | will cluster around the true age. But we have to continue to worry |
|
| 201:57 | this problem of uh if we see like that and we're interested in the |
|
| 202:01 | of crystallization, we're gonna zap it the corner here. If we're interested |
|
| 202:05 | the old part, we go for . Um got a few minutes and |
|
| 202:14 | so, oh we got a That's a whole another thing. |
|
| 202:17 | no, no. So we got , I'm gonna stop here because my |
|
| 202:20 | is going and this mono is another story that we're gonna have to talk |
|
| 202:24 | . So tomorrow morning, we will here and we'll finish the uranium lead |
|
| 202:31 | and then we'll go on to the argon stuff, which is already loaded |
|
| 202:36 | . I'll have to, I'm gonna on these slides. I've still, |
|
| 202:39 | basically don't have to do much, I'm gonna, I'll load up some |
|
| 202:42 | slides tomorrow that or I'll send them we'll get them loaded up. So |
|
| 202:46 | can download those tomorrow morning. any questions? All right, we |
|
| 202:56 | carry on with more dating of uranium , uh, in the |
|