| 00:00 | tell me about yourselves. Hello, name is David, your friend. |
|
| 00:20 | missed my uh away. Okay. . That helps. Um So I |
|
| 01:04 | I can start by sharing my Let's see if I can find it |
|
| 01:08 | here. Our .1. There we . So I put these, these |
|
| 01:16 | on blackboard yesterday. I don't know you've noticed it, but I put |
|
| 01:21 | whole bunch of different um I think about six or seven different versions, |
|
| 01:27 | files that are on blackboard now, the one we're going to look |
|
| 01:32 | So if you want to go to and get those, you can do |
|
| 01:36 | now or at some other time. the this this uh or shrimp, |
|
| 01:51 | screen sharing is paused. What does mean? Is that a problem? |
|
| 02:08 | , no resume machine. The pain stop sharing sharing. Okay. Um |
|
| 02:51 | . So now it's, it's, now it's it's hung up again. |
|
| 03:17 | . Oh, she's the host. should be the host. Can I |
|
| 03:24 | the host? Usually not, but I give you give you a ride |
|
| 03:49 | . It's a fun salt. I'm stop sharing and start again. Should |
|
| 03:55 | wait or go ahead construct share share screen. Okay, that's |
|
| 04:10 | Okay. So what we're gonna do these 2 3 days that we'll be |
|
| 04:19 | is talk about how we put a millions of years on things uh, |
|
| 04:28 | to take it a little further to more than just millions of years on |
|
| 04:33 | . But in general when we date probably isotopes, we don't really date |
|
| 04:39 | age of the mineral. In some cases we do. But what we're |
|
| 04:44 | doing is measuring the last time the was a closed system, we'll talk |
|
| 04:50 | about what that is. But the system generally has something to do with |
|
| 04:56 | . And so when we data mineral telling, we're learning when it was |
|
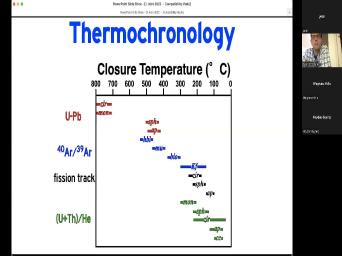
| 05:00 | at a particular temperature more or And um this this um shows the |
|
| 05:09 | of temperatures that we can understand using , different um Different dating schemes and |
|
| 05:16 | minerals. We'll talk about uranium, dating, Argon 40 39 dating, |
|
| 05:21 | track dating, helium dating. We'll about a few other things as |
|
| 05:25 | And you can see that different minerals be applied in each one of those |
|
| 05:31 | And depending on which mineral you you may be learning about when the |
|
| 05:35 | was at 500° or 400 or 300 200 or less than 100. And |
|
| 05:41 | you got to know which is which talking about. Um If for example |
|
| 05:46 | had a pile of documents, you , you have documents at home about |
|
| 05:50 | life and they all give different You know, you may have your |
|
| 05:54 | certificate and the day you graduated from school and the day you graduated from |
|
| 05:59 | , maybe your marriage certificates in there all these different things and someone might |
|
| 06:04 | at that and say, well there's problem here, all these dates are |
|
| 06:08 | . But if you understand that the pieces of paper are recording different |
|
| 06:13 | then it's not a problem at In fact, it's very useful. |
|
| 06:16 | say, Oh well you won't. graduated from, from college only one |
|
| 06:20 | after graduating from high school. That's interesting. Or you know, it |
|
| 06:24 | you 20 years to graduate from high . That's information because we understand what |
|
| 06:29 | one of these documents mean. We understand what each one of these dating |
|
| 06:35 | mean. When we date something, the Argon 4039 system in Muscovite. |
|
| 06:39 | learning about when the rock was around when we dated using fission track on |
|
| 06:46 | . We're learning about when the rock at 100. Now, why do |
|
| 06:52 | change temperature? Different processes such as , weathering will cause a rock to |
|
| 07:07 | what? It's really, I can't either one of you, maybe we |
|
| 07:18 | move up to here or this room there's, Would you mind taking these |
|
| 07:24 | seats, would that be hard? crazy to have just two people and |
|
| 07:39 | spread out as much as we Thank you for doing this. So |
|
| 07:54 | erosion, how does that cause rocks change their temperature get hotter? They |
|
| 07:59 | older, They stay the same. that will affect the rate, but |
|
| 08:09 | the direction they get hotter or Explain how that works. Oh |
|
| 08:23 | that doesn't heat up rocks much at . I mean, not, |
|
| 08:27 | not compared mean why when we go , what happens? It's hotter down |
|
| 08:34 | ? Right, so that's what we're talking about. That's the temperature. |
|
| 08:39 | not talking about this, we're talking the fact that eventually it's melting |
|
| 08:44 | it's hotter down. Uh So when have corrosion taking place now, here's |
|
| 08:53 | rock, it's in, it's in crust, what can happen to |
|
| 08:58 | Can heat up or cool down? , what's gonna cause it to cool |
|
| 09:06 | ? But saying it's a depth of km right now, water could |
|
| 09:14 | a little bit, a little What temperature is it down there at |
|
| 09:18 | km? No idea. You have gradient, you ever heard of |
|
| 09:28 | Got a number for that? 30, 40°/km2 I'm are you going |
|
| 09:37 | be bothered by degrees Celsius per A lot of people who are in |
|
| 09:42 | oil business when they talk about degrees 400 ft. Um We're gonna go |
|
| 09:48 | C per kilometer. It's about 25 just say 25 degrees C per |
|
| 09:54 | It could be 32 B 35 B let's just say 25. Nice number |
|
| 10:00 | talk about. So, what's the at five kilometers below the surface? |
|
| 10:05 | , maybe you would add some temperature the surface, you know, it |
|
| 10:09 | be 145. So Rocks down there a 150° Is it always going to |
|
| 10:19 | at 150°. What could make it change ? Yeah, how and and and |
|
| 10:32 | do you mean by uplifting uplift relative what? Well, a fault? |
|
| 10:40 | not really the, that's not the that cools things down. Why? |
|
| 10:44 | , why, why does it get up here because it gets closer to |
|
| 10:47 | surface? Right, faulting can do . But let's just think in broader |
|
| 10:53 | . All I'm looking for here is when you get close to the |
|
| 10:56 | you should get colder when you get away, you should get hotter. |
|
| 10:59 | causes you to get close to the ? Would be erosion up here. |
|
| 11:04 | . What cause did you get farther from the surface? Slightly more |
|
| 11:11 | but not much. I mean, the opposite of erosion burial, but |
|
| 11:16 | can also get hotter by not just processes by structural processes. We could |
|
| 11:22 | fault, thrust faulting which would cause rocks to become very deeper. In |
|
| 11:28 | case the rocks are changing temperature. if we can understand when they change |
|
| 11:35 | and how fast we can understand what are going on. Is this a |
|
| 11:41 | belt? This is a base in . Okay, and then, so |
|
| 11:46 | that's why all these closure temperatures, these different temperatures are valuable because each |
|
| 11:50 | tells us about a different thing and a rock may not the most |
|
| 11:57 | cooling time for Iraq might not you know, might might only be |
|
| 12:02 | to us by two of these and have to understand which two. |
|
| 12:08 | and then from the perspective, you're interested in oil and gas business, |
|
| 12:14 | temperatures are important from the oil and perspective, what you need to make |
|
| 12:27 | . Nobody's told you that yet. , it's maybe very bottom line would |
|
| 12:33 | sort of 70°C. The rocks aren't buried 70 or 80°. Nothing happened. And |
|
| 12:42 | you can go too far, you up above 160 or 70°. It's all |
|
| 12:48 | up. That's what's called the oil . You heard of something called the |
|
| 12:52 | window? That's a temperature window which of course can be correlated to |
|
| 12:57 | depth. But there are techniques in that will tell us about a past |
|
| 13:04 | . We don't have good, we have good methods for telling us about |
|
| 13:09 | past depth. Not as many And really from the point of view |
|
| 13:13 | thermal thermal maturation of hydrocarbons, temperature the thing that we worry about temperature |
|
| 13:19 | related to death and it's not always same depth, but temperatures the important |
|
| 13:23 | . So, that's introduction. So we get into some of that, |
|
| 13:28 | going to go through some basic some some, some math, some |
|
| 13:32 | about what we need to know. then we'll then we'll go through several |
|
| 13:38 | ways in which we date things uranium basically. We'll talk about in |
|
| 13:43 | we'll talk about the ways in which decays and the way in which potassium |
|
| 13:47 | uranium. We get three different potassium is also very valuable because lots |
|
| 13:53 | minerals have potassium. Um and we'll about some other stuff. So starting |
|
| 14:02 | simply, we've got the principle of rocks on top are younger than the |
|
| 14:07 | on bottom and that's a good place start. But and that and that's |
|
| 14:11 | it took to come up with the time scale because the geologic timescale was |
|
| 14:16 | together without any understanding of those numbers the side. Right. They decided |
|
| 14:20 | these rocks are Jurassic because they're on of these other rocks which are |
|
| 14:24 | And we're going to mark this boundary the Jurassic and the Triassic based entirely |
|
| 14:29 | what kind of fossils are. But sometime in sometime later, somebody came |
|
| 14:34 | and said that Palin to logically interesting is 214 million years old. How |
|
| 14:40 | they figure that out? Well, consider one of these geologically interested paleontological |
|
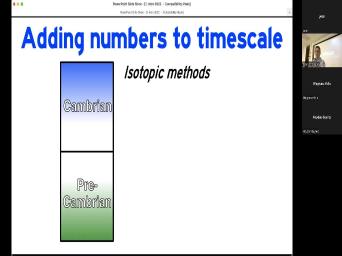
| 14:46 | things. And as I said, we can talk about the Cambrian pre |
|
| 14:49 | and that will be unchanging. We've on what the Cambrian fossils are. |
|
| 14:54 | not a controversy and that's defined from fossils. It's not going to change |
|
| 15:00 | it's not. And the same thing whether you're talking about Cambrian pre Cambrian |
|
| 15:04 | legacy. Eocene or any of these . These are based on what the |
|
| 15:09 | look like. Okay, But then are we going to know how many |
|
| 15:13 | ago? That one? How could find out? Fair enough, |
|
| 15:27 | Well, carbon dating almost no we'll talk about this soon. But |
|
| 15:33 | 14 has a half life of 5700 . That's not very long. So |
|
| 15:41 | , not carbon too fast, but . Yeah, sure. But what |
|
| 15:48 | would we date? You're a manager . You can tell me I have |
|
| 15:51 | go collect a sample. What do want me to get to figure out |
|
| 15:55 | ? There's some, some geologic circumstance would be advantageous to this problem right |
|
| 16:02 | , we've just got an outcrop that's some fossils there. What would you |
|
| 16:07 | would be your dream case of place we could learn something about the age |
|
| 16:11 | that boundary there layers here. How are they? Well, here's some |
|
| 16:18 | . What if we had a The crosscut situation? We can date |
|
| 16:26 | rocks. You knew that? So we could date an igneous |
|
| 16:30 | We crossed this dike cross cuts the . That's good. Right. We |
|
| 16:35 | date that. But what will that us about the boundary? We did |
|
| 16:40 | red cross cutting thing. What do now know about the boundary? Not |
|
| 16:53 | . Sorry, that kind of Older. Right. All we know |
|
| 16:58 | it's older than that. Now, we're looking at the canyon. We |
|
| 17:02 | this is Cameron pre Cambrian, somebody a Dik Dik Dik the ducks 100 |
|
| 17:07 | years old. Well now we know the Cambrian pre Cambrian boundary is less |
|
| 17:12 | 100 million. We know that if can do better than but that's all |
|
| 17:17 | can, you know now, if got lucky and found a diet that |
|
| 17:20 | 543 million years old. So that going to narrow it down. But |
|
| 17:26 | , that's that's that would be Okay, let's do that. Another |
|
| 17:31 | would be to look at look at minerals in the sand. Let's say |
|
| 17:35 | is a sandstone. It's Cambrian We could look at the minerals in |
|
| 17:39 | and we could date them individually Geo chemically, that's not a big |
|
| 17:44 | . We can date one mineral at time. Let's gather a bunch of |
|
| 17:48 | grains and let's let's say there's your . But they could be lots of |
|
| 17:53 | . But let's just say there's your will measure the zircon ages. That's |
|
| 17:57 | all the time. Now, what that tell us about the age of |
|
| 18:01 | Cambrian sandstone please. Yeah, but that's that's a general answer. You |
|
| 18:17 | me a specific but wrong answer a ago. Try and give me give |
|
| 18:21 | another specific answer. It's just like last answer. It's one of those |
|
| 18:27 | things. Is it younger you said we date the dike. We know |
|
| 18:32 | know that the rock must be younger the dike. Right, what does |
|
| 18:37 | tell us those minerals existed before the . Right, so the sandstone can't |
|
| 18:55 | until that they are deposited. The have to always be older than the |
|
| 19:00 | itself. So this and when we these minerals in here, suppose we |
|
| 19:05 | we did individually minerals, suppose we 100 different minerals. Which one is |
|
| 19:11 | most important for this for this problem . You just knew it was the |
|
| 19:31 | one. I got all these great got a sandstone. What if I |
|
| 19:39 | down to the beach in Galveston this we got a bunch of grains and |
|
| 19:43 | dating them. Say I got a years and 500 million years and 40 |
|
| 19:49 | years. What does that tell us the age of the stand down |
|
| 20:00 | How can this? We could Sure go ahead. Um We got |
|
| 20:12 | we got a sandstone, it's got in it, all of them are |
|
| 20:17 | than the fan stone. Right. so if we have an age of |
|
| 20:23 | grains sandstone is older or younger, , youngest one will get us to |
|
| 20:33 | best possible estimate we get if we a million grains from the sandstone, |
|
| 20:38 | one we're Keenest on is the Oh what have we done here? |
|
| 21:18 | got nothing here? Well I mean was I can't get to my, |
|
| 21:38 | is locked up now because you extend window too. Oh it's a whole |
|
| 21:46 | over here. I see. so now I gotta no first come |
|
| 22:11 | . Alright. If I can just it to mirror displays will be |
|
| 22:23 | Uh arrangement mirror displays. Okay, now we go back to zoom still |
|
| 22:35 | that share screen back to this. , no, no don't do |
|
| 22:42 | do that. Uh Where did it ? This 1? Yeah, it's |
|
| 22:54 | one. All right. He did . Where'd you get this from? |
|
| 23:08 | . S. M. I. . They always come through, don't |
|
| 23:14 | ? That's fine. We got Okay and so we have to give |
|
| 23:22 | back to them but we need it . Can we borrow it till |
|
| 23:29 | Go ask them, please tell them I can deliver it to them on |
|
| 23:36 | or you Well one of us Okay, so we've got uh this |
|
| 24:00 | Oh she's paused, presume share. gonna stop share and start over. |
|
| 24:13 | seeing anything now. Okay, that's . Okay now we go to where |
|
| 24:24 | it? Uh Not that one, one. Yeah, this one got |
|
| 24:34 | . Okay. All four things are same now. Alright, great. |
|
| 24:37 | , so we're talking about this to dating again. We got 100 grains |
|
| 24:43 | there. We we yeah, We've the youngest one is the most important |
|
| 24:50 | . Now if I if I date sandstone and I get a bunch of |
|
| 24:54 | and then the the youngest one is million, This tells me that the |
|
| 24:59 | has to be less than 92 Does't have to be a lot |
|
| 25:03 | but it could it could be 92 , but that's the maximum deposition |
|
| 25:08 | Okay, so that's another way to numbers to the timescale. Um a |
|
| 25:14 | way, the best way, the way to date a sedimentary rock is |
|
| 25:18 | date an igneous rock like this. , you know, this is not |
|
| 25:23 | option. Wouldn't it be nice if was a highlight underneath every sandstone? |
|
| 25:29 | this is just, you know, when we talk about where all those |
|
| 25:32 | for the timescale went, it's when were able to find this, this |
|
| 25:36 | the best and why is it Because you've got these two things |
|
| 25:41 | these two and volcanic rocks are the rocks to interpret basically of all |
|
| 25:48 | A volcanic rock is something that goes very hot, very cold, very |
|
| 25:53 | . We don't have to worry about deposition environment or how long it took |
|
| 25:56 | accumulate this stuff or or what the or pressures are. We know this |
|
| 26:01 | at the surface of the earth, happened quickly. And so let's date |
|
| 26:07 | . And so we can and we'll about the techniques, we used to |
|
| 26:09 | them, But wouldn't it be nice we did this? And what would |
|
| 26:12 | even better if you could find right? And that's what has moved |
|
| 26:18 | . Our understanding of the timescale is and more times. People find |
|
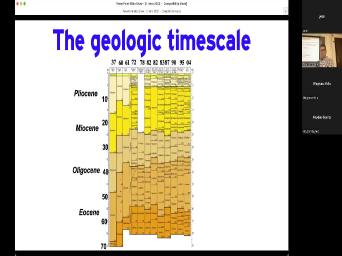
| 26:23 | Okay. This illustrates that by looking the geologic time scale from 72 to |
|
| 26:31 | , uh based on different publication dates 1937. They thought the boundary between |
|
| 26:39 | and the was about 48 million years we've come to think is at 34 |
|
| 26:45 | 3.6. Look at all that changing that's just in the in the in |
|
| 26:50 | , In the sentence in this, the Nea gene, right? You |
|
| 26:55 | draw the same sort of graph for these other things and they're up and |
|
| 26:57 | , back and forth. When I an undergraduate, I was told that |
|
| 27:01 | boundary between the pre-cambrian? The Cambrian 570 million years. Now we tell |
|
| 27:07 | undergraduates that is four, million. that's because they eventually found a better |
|
| 27:13 | of these. Right? So that us to just how old things |
|
| 27:21 | What is, how old is the ? How old cases? And we're |
|
| 27:25 | talk a lot about that. But we'll also talk about ways in which |
|
| 27:29 | have to be worried about, you , you get an age, you |
|
| 27:32 | a sample, you dated, how that age be goofed up? What |
|
| 27:35 | the concerns? What are the, are the pitfalls generally they are, |
|
| 27:40 | the rock been heated up? or to turn it around. We |
|
| 27:45 | the rock's been heated up. Take of that to tell us something about |
|
| 27:49 | deposition or tectonic history. Well, know all sorts of things about the |
|
| 27:56 | based on for lots of other mountain . The Himalayas more than many. |
|
| 28:00 | I've worked in the Himalayas. So used that as an example. But |
|
| 28:05 | ranges, Himalayas, the alps, lot of them. We know about |
|
| 28:08 | they've come up because we can look the rocks and say, well this |
|
| 28:11 | was at this temperature then. And this temperature in this temperature and you |
|
| 28:15 | a history of temperature versus time and go something interesting is happening right |
|
| 28:20 | Uh similarly you can watch the grains of the mountains and into the basins |
|
| 28:25 | you can watch the basin show up tell when it got hot or did |
|
| 28:28 | get ever? Uh So there's the . So how do we add numbers |
|
| 28:36 | this strata? Graphic column, which based on fossils. We're going to |
|
| 28:39 | it on the decay of radio Um And as I said, it's |
|
| 28:44 | capable of providing information about thermal And I said this, I talked |
|
| 28:49 | uplifted mountains already. You can also this to understand the timing of structural |
|
| 28:55 | in particular faulting. Um And you calculate rates of deposition from fossils usually |
|
| 29:03 | fossils aren't always present. So we add strata graphic concerned here and then |
|
| 29:07 | the basin analysis part I mentioned to can sometimes get a sense of what |
|
| 29:12 | maximum temperature of the basin was sometimes even the duration of that. And |
|
| 29:18 | T max and duration are the two that go into whether or not |
|
| 29:23 | you've turned, you know, sludge oil. So we'll return to geology |
|
| 29:30 | . We're going to talk about chemistry physics and math for a little |
|
| 29:34 | Um, you've had calculus. Have had differential equations? Yes. |
|
| 29:40 | Okay, that's fine. Um, had chemistry, we've got electrons, |
|
| 29:49 | and neutrons, we know all about and we talk about the atomic number |
|
| 29:53 | a the number of protons and his of neutrons and Z is the to |
|
| 29:56 | together. And then there are Isotopes are atoms of the elements with |
|
| 30:01 | same number of protons. They may have different but different number of |
|
| 30:06 | So uranium 235 Iranians are both isotopes uranium. Those two both happen to |
|
| 30:12 | radioactive Strontium 87 and strontium 86 ri neither one of them are radioactive but |
|
| 30:21 | 87 is radio. It is the daughter product of Rubidium 87. So |
|
| 30:29 | 2 35 and 2 38 are getting all the time. Strontium 87 is |
|
| 30:35 | bigger all the time. And strontium is not changing. Carbon 14 as |
|
| 30:42 | mentioned a minute ago. That's But it gets in the news all |
|
| 30:46 | time because it's what happens when they some sort of, you know, |
|
| 30:50 | trout of touring or something like that archaeology. You can date stuff with |
|
| 30:55 | , but only if it's less than 20,000 years old. If you want |
|
| 30:59 | date a rock, you want to the cretaceous. No good. Carbon |
|
| 31:03 | , another isotope of carbon, not act. The only difference between carbon |
|
| 31:09 | and carbon 14 is two protons. that makes one of them radioactive. |
|
| 31:15 | so when we say that things are , refer to them as the |
|
| 31:19 | the things that they decay to are daughters. Um, eventually in all |
|
| 31:25 | you will find a stable daughter. it is not the case that it |
|
| 31:30 | immediately. Sometimes a radioactive thing will to another radioactive thing and another and |
|
| 31:35 | and another. And then finally, in the case of uranium 2 35 |
|
| 31:39 | 2 38 it can be seven or different steps until you finally get to |
|
| 31:43 | stable thing lead. Um, the at which this happens is a constant |
|
| 31:52 | . The half life of these of approaches are constant and we know what |
|
| 31:56 | are. Um, so if we the rate of decay, we know |
|
| 32:01 | amount and we can measure in the the amount of parents, the amount |
|
| 32:04 | daughter, We can just straightaway calculation figure out how long the system has |
|
| 32:09 | acted. Um, how do things ? There's a bunch of them will |
|
| 32:14 | through them quickly. It's not really . It's a little bit important in |
|
| 32:18 | cases. Uh One thing to point is the type of decay does not |
|
| 32:22 | the rated K. You can have alpha decay, slow alpha decay. |
|
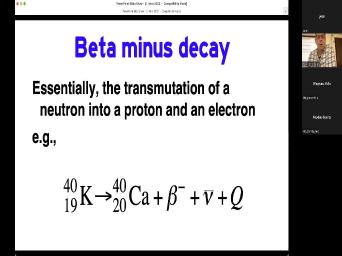
| 32:26 | not a thing. Um Let's start beta minus decay. That's a transmutation |
|
| 32:32 | a neutron into a proton. And electron an example here would be potassium |
|
| 32:37 | decays to calcium 40 plus this uh particle plus a neutrino plus energy. |
|
| 32:47 | notice how these things are written 40 K 40 is the atomic number. |
|
| 32:53 | the number of protons plus neutrons, is the number of protons. You |
|
| 32:58 | often see it written like that because means 19 protons. There's no other |
|
| 33:05 | besides potassium that has 19 protons. that's totally redundant. But it's nice |
|
| 33:10 | to do it this way because we see what's going on. We go |
|
| 33:13 | potassium 42 calcium 40. How did happen? Well, the 19 went |
|
| 33:17 | 20. Yeah, because we have have taken a neutron and turned it |
|
| 33:24 | a proton and then we we we out this beta particle but inside the |
|
| 33:29 | inside the nucleus we trans we have a neutron and made it into a |
|
| 33:34 | . And that's why this number didn't . But this one and that's what |
|
| 33:38 | call it calcium. Okay. Um a picture of it. There's, |
|
| 33:44 | know, things go on inside and , comes and out comes this this |
|
| 33:55 | . Yeah, but it doesn't reduce total neutrons plus protons. That's |
|
| 34:01 | you see, there is no there no neutron only number here. This |
|
| 34:06 | protons, protons plus neutrons. Because went down, that other one had |
|
| 34:11 | go up. Yeah. Um here's one that's not real important and a |
|
| 34:19 | unimportant example here. But flooring 18 decay to oxygen 18. And here |
|
| 34:25 | go. Here we go. In opposite, we're taking a proton and |
|
| 34:28 | it into a neutron. Um that can happen. Um another example, |
|
| 34:36 | me see. Do I have I don't I don't have it written |
|
| 34:39 | here. But here's this is a take a take a capture an electron |
|
| 34:51 | create a neutron from a proton. sometimes follow this, the began to |
|
| 34:58 | the action. And one example is 14 comes out. Uh or |
|
| 35:11 | no, no, I'm getting ahead us. Electron capture An example of |
|
| 35:15 | capture is not shown here. I'm , would be uh argon potassium 40 |
|
| 35:26 | to argon 40. I'm sorry. mixed that. We'll get back to |
|
| 35:30 | a bit then. The the last time here is alpha decay in which |
|
| 35:35 | have a two protons and two neutrons from the nucleus. And so here's |
|
| 35:44 | example of uranium 2 38 case story 34 plus. It's called an alpha |
|
| 35:50 | because this thing here is called an Barda. This is actually going to |
|
| 35:54 | the basis of one of our dating on the accumulation of these guys. |
|
| 36:00 | in this case story into 34 is radioactive. It's going to decay to |
|
| 36:04 | else. Bottom example Samarian 1 47 morning 43 that's one alpha decay. |
|
| 36:12 | stable. We don't do anything There's a picture. You've got |
|
| 36:17 | 4 things leaving here. Okay, is what I was getting at |
|
| 36:23 | The N. P. The P. Reaction is when you have |
|
| 36:27 | neutrons can displace the proton from the and this happens in the atmosphere all |
|
| 36:32 | time when cosmic rays come at neutrons from stars. Somewhere in the |
|
| 36:38 | come zinging at us, they hit nitrogen atom and that embeds the neutron |
|
| 36:44 | the atom and a proton gets thrown . That changes nitrogen 14 into carbon |
|
| 36:50 | . And now carbon 14 is Um This happens in nature. This |
|
| 36:56 | something we'll talk about tomorrow morning. happen in nature but we use it |
|
| 37:00 | our advantage when we do this, dating stuff, we can artificially produce |
|
| 37:06 | 39 by taking passing 39 putting it a nuclear reactor, throwing some neutrons |
|
| 37:12 | it and the proton comes out. make argon 39. We'll talk tomorrow |
|
| 37:16 | why that's a good idea. Ah then one more thing I think is |
|
| 37:21 | last thing is another kind of decay which instead of throwing off little bitty |
|
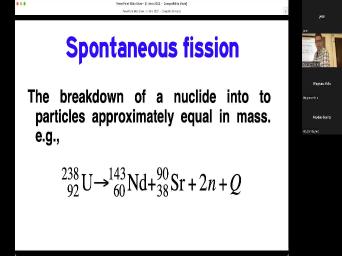
| 37:27 | like an electron or even an alpha , a big nucleus will actually break |
|
| 37:32 | two different pieces that are about the size. Here's an example of uranium |
|
| 37:36 | 38 breaking up into 1 43 and 90. Those two things are pretty |
|
| 37:43 | , relatively speaking. And then the energy of this thing causes them |
|
| 37:47 | move away from each other. Um should point out that this is just |
|
| 37:52 | of the many ways in which uranium 38 can efficient. It doesn't always |
|
| 37:57 | neodymium 1 43 and strong and They're just always two pieces that are |
|
| 38:02 | the same size, you know, 43 and 90. They're not exactly |
|
| 38:05 | same size but they're both big. this is the basis of one of |
|
| 38:09 | dating systems which is called fission track . And that's when you have a |
|
| 38:14 | like zircon or appetite, you have uranium 2 38 in it. And |
|
| 38:19 | these things just break into two pieces they will move in the crystal |
|
| 38:24 | they'll move about 15 microns and through distance the lattice is disrupted and there |
|
| 38:31 | a what's called efficient track. It's it's a new physical change in the |
|
| 38:38 | and that makes it different from all other systems where we're measuring some |
|
| 38:44 | we measure the amount of lad in amount of the amount of uranium with |
|
| 38:47 | sort of chemical gizmo here, we get out of microscope and we say |
|
| 38:53 | one of those little tracks. That's one. You count them up like |
|
| 38:58 | . That is the daughter product in case is the fishing track. It's |
|
| 39:02 | don't measure uranium decays to need DM . We don't measure that thing. |
|
| 39:07 | just measure the consequence of them moving . Um the only ones that we |
|
| 39:15 | pay attention to are the big I mean it says all nuclei with |
|
| 39:19 | above 100 are unstable with respect to , but they happen so unbelievably slowly |
|
| 39:24 | even uranium 23 to 38 has a , really, really long half |
|
| 39:29 | The only thing we're going to pay , I mean theoretically other other atoms |
|
| 39:35 | condition the only one we're going to attention to is uranium 2 38. |
|
| 39:41 | , so radioactive growth is radioactive Growth decay. Good news. It's |
|
| 39:49 | of temperature pressure. This is wonderful , is it not? Because if |
|
| 39:53 | had to worry about the king's decaying slowing down and speeding up, depending |
|
| 39:57 | whether they were buried or that's not thing. Good news. Um It |
|
| 40:05 | also a thing that when you if were to put a pound of potassium |
|
| 40:10 | the on the table here, There's about an individual potassium 40 atom. |
|
| 40:15 | could look at it and say it's ready about ready to go. You |
|
| 40:19 | , you can tell which which tomato about to fall off the off the |
|
| 40:23 | , but not which which potassium item about to become an argon at just |
|
| 40:28 | just once in a while they But what you can say is that |
|
| 40:33 | I have a pound of potassium or kilogram of uranium assert, I can |
|
| 40:39 | how many of them will decay in given amount of time next year. |
|
| 40:44 | many will decay. And you come a year later and sure enough, |
|
| 40:47 | how many have decayed. I can't you which ones, but I can |
|
| 40:50 | you what the proportion will be. that's because each of them has a |
|
| 40:56 | of decay next year. And if have a million of them, you |
|
| 41:00 | ? And and so that's how that's that works because probability of decay. |
|
| 41:07 | we can then talk talking about that more mathematically, we can think of |
|
| 41:11 | probability of decay in some small time DT is going to be um lambda |
|
| 41:19 | where lambda is this proportionality of how to decay. And we can, |
|
| 41:27 | can, we can say that this then is more likely to occur. |
|
| 41:32 | rate of change in the number we is gonna be proportional to the number |
|
| 41:36 | have. It's uh it's a small , I don't usually have two people |
|
| 41:42 | use this experiment, but if, know if you were to get some |
|
| 41:45 | and flip a coin how many heads would would this class likely to get |
|
| 41:50 | ? Right. I mean two is a good number to do but let's |
|
| 41:54 | just say one. Well let's say had a class of 20 people would |
|
| 41:58 | 10 heads right Now let's go down Astros game tonight. There'll be like |
|
| 42:04 | people watching a baseball game tonight. asked them all to get out of |
|
| 42:07 | and flip their quarters. How many are we going to get? |
|
| 42:14 | But does that mean that those coins different down there at minute Maid |
|
| 42:18 | No they're exactly the same coins. the reason we got more heads is |
|
| 42:22 | we had more flips but we couldn't ahead of time which one was gonna |
|
| 42:26 | a head which is going to be tail. We just did it. |
|
| 42:28 | then okay And then if we asked at the baseball game, Okay all |
|
| 42:33 | people who got tails sit down. with heads flipped again. We should |
|
| 42:37 | get 7000 heads. And next time 3000 heads. And then only. |
|
| 42:42 | each time we go through this the of people sitting down become fewer and |
|
| 42:47 | . That has nothing to do with probability of decay. It has to |
|
| 42:50 | with a number of things that might . And that's why we can write |
|
| 42:54 | as this. the the rate of D. N. D. |
|
| 42:58 | Is proportional to end We have a decay rate. If we have a |
|
| 43:02 | of once we get down to a like this the decay rate is one |
|
| 43:07 | but when we went to the Astros the decay rate was 15,000 but that's |
|
| 43:13 | not a great equation but we can that into a week. That isn't |
|
| 43:16 | equation it's proportionality. But we can it into an equation by putting this |
|
| 43:21 | probability of individual. Okay so now got an equation that says that the |
|
| 43:27 | of decay is equal to the number have times the probability of an individual |
|
| 43:35 | . Okay um We just rearrange that integrate. We get a nice simple |
|
| 43:40 | that says the log of an equals T. Plus some constant T. |
|
| 43:45 | the time involved. Okay well that's . Um If we take the amount |
|
| 43:53 | parents present at T. Zero to n zero then we can evaluate what |
|
| 43:59 | constant of integration is and we'll end going through you know it's a simple |
|
| 44:04 | for there. We get we get equation that says that and over and |
|
| 44:10 | is either minus lambda T. Or can rearrange that. We can solve |
|
| 44:15 | for end the number of parents we right now or we can solve it |
|
| 44:20 | and not the number of parents we . Okay what good is that? |
|
| 44:25 | let's let's take note of the fact when we have parents were making daughters |
|
| 44:31 | so we can think that the number daughters is going to be equal to |
|
| 44:35 | number of parents we started with minus number of parents we have. |
|
| 44:39 | Now, I should point out that this star here means the number |
|
| 44:43 | radioactive daughters, it could be that are daughters to begin with. We're |
|
| 44:50 | to ignore that for the moment. that's what this D. Star is |
|
| 44:55 | . The number of daughters you measure the number of daughters who started to |
|
| 44:59 | with the non. Okay. But we're gonna make this substitution to get |
|
| 45:05 | of R and not make that We start, you know, we |
|
| 45:09 | this end on up here, we here, we get an equation that |
|
| 45:14 | becomes, it comes this one down In which now we have an equation |
|
| 45:19 | the age of our system which is in terms of time variable is a |
|
| 45:26 | . Is the constant for the system involved with. It will be a |
|
| 45:30 | number whether we're talking about potassium 40 uranium 235 or carbon 14. That |
|
| 45:36 | constant, but but a system specific . And then and and er the |
|
| 45:43 | of parents in the number of guards we've got this problem here is that |
|
| 45:47 | need to know how many daughters we . That's probably okay, how we |
|
| 45:52 | get around that. Well that's the the fundamental age equation. So when |
|
| 45:58 | talk about the age equation, that's beginning of it. And sometimes we |
|
| 46:02 | work with this do not. Sometimes can't. But before we talk about |
|
| 46:06 | I want to take a moment just make sure we understand another concept which |
|
| 46:09 | the half life. And so the life of a radioactive isotope is the |
|
| 46:14 | required for half of it to decay . This is a constant amount of |
|
| 46:19 | , not a constant amount of Right? If we were using American |
|
| 46:26 | , the half life of getting from from you know a number of number |
|
| 46:31 | coins. If we if we eliminate the tails every time, the half |
|
| 46:35 | to get down to half as many be one flip right every time you |
|
| 46:39 | halfway, if we were rolling some of fancy, you know dungeons and |
|
| 46:43 | dies with 20 sides on it. we said we only we only get |
|
| 46:47 | of people when they roll at 20 wouldn't be one. It would be |
|
| 46:50 | bigger number. Okay. But it's the not it's not it's the time |
|
| 46:57 | takes to go in half not to not the time it takes to decay |
|
| 47:01 | certain number. The same number going a half to a quarter as from |
|
| 47:05 | quarter to an eighth. Alright. at at T 11 half. If |
|
| 47:10 | don't worry about the beginning number of at T. One at this time |
|
| 47:15 | number of daughters equals the number of equals. And we can substitute that |
|
| 47:19 | there and then we can rearrange and can find out that the half life |
|
| 47:24 | that is just the log of two by the decay cost, whatever that |
|
| 47:30 | for your system. Carbon or carbon or uranium or whatever. |
|
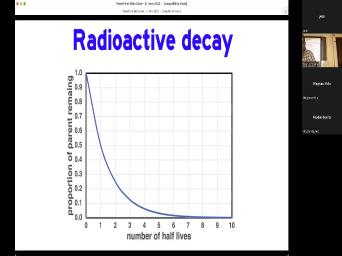
| 47:35 | That's what a half life. If if we look at half life autograph |
|
| 47:40 | decay on a graph, this is is applying that equation and we can |
|
| 47:45 | that after if we start with 100% something uranium or potassium after one half |
|
| 47:51 | , we're down to 50% of After two half lives were down to |
|
| 47:56 | and so forth. You'll notice that about four half lives, we really |
|
| 48:03 | want to deal with it anymore because how flat that curve is. And |
|
| 48:08 | you see the analytical challenges have occurred that slope. Trying to understand what |
|
| 48:14 | value is after five half lives and it from after 45 half lives, |
|
| 48:21 | not much difference, right? So , unless you can make a perfect |
|
| 48:26 | , you should stay away from systems have gone past four half ones because |
|
| 48:31 | got down to that point where it's impossible to to really get it |
|
| 48:36 | And as a matter of fact it's and it's not just, you're not |
|
| 48:41 | , well, it's, it's hard a, from that sort of slope |
|
| 48:46 | , but it's also hard because one the things you're trying to measure is |
|
| 48:49 | gone. So This is why we use carbon 14 to date old things |
|
| 48:55 | it has a half life of less 6000 years. So from by |
|
| 48:59 | by this graph, we should stay from things that are 30,000 years |
|
| 49:03 | Okay. From the same point of , radioactive growth is the opposite. |
|
| 49:08 | should try and stay away from things have only gone through about 1/10 of |
|
| 49:11 | half life because we're on the steep of that curve. And it's also |
|
| 49:15 | to tell the difference between a 10th and thousands. Okay, um, |
|
| 49:25 | I said, some daughter products are radioactive, but ultimately you'll find something |
|
| 49:30 | stable. Here's the decay series of 2 38 it goes back and |
|
| 49:35 | back and forth and eventually it gets the lead to six, no matter |
|
| 49:39 | you do, it'll get down Okay, um, you've heard of |
|
| 49:46 | called uniformity Arian ism. I imagine present is the key to the past |
|
| 49:50 | one way to look at that. uniform materialism can go too far. |
|
| 49:55 | it really means is natural laws don't however, the rates and intensities or |
|
| 50:00 | may change, uniform materialism shouldn't be so far as to say, |
|
| 50:05 | it's a beautiful day outside today. always that way. You know, |
|
| 50:10 | we, if we practiced strict uniform , we would never understand why the |
|
| 50:14 | went extinct because there are no meteorites out of the sky today or |
|
| 50:19 | Hurricane Harvey tells us not to be uniform a terrian ism because one |
|
| 50:25 | one weekend it rained 60 inches. was unusual. But it wasn't |
|
| 50:31 | it wasn't a breaking of the Water still flows downhill those days. |
|
| 50:35 | was more water that day. But , so that's the sort of lacks |
|
| 50:40 | vegetarianism. When it comes to rate , we have no room for |
|
| 50:46 | The key. The big assumption of this is that the half lives of |
|
| 50:50 | isotopes today are the same. They billions of years ago. If this |
|
| 50:54 | not the case, then when we our, when we put lambda in |
|
| 50:58 | , you know, we'd be using wrong number. If there was an |
|
| 51:01 | lambda, that was different back what was it? Was it |
|
| 51:05 | Smaller. This is a terrible thing have to worry about. And the |
|
| 51:10 | news is, we don't have, don't think there's anything going on |
|
| 51:14 | And one of the ways that we've this is looking at meteorites and moon |
|
| 51:19 | , we have an idea of the lives of these things and you can |
|
| 51:23 | that they fear a whole bunch and rocks. Moon rocks are great because |
|
| 51:34 | have such simple history, especially if volcano across their assaults on the |
|
| 51:40 | Right? We've got someone from down road just down here. Probably got |
|
| 51:45 | of them over in the next Uh, what's so nice about |
|
| 51:50 | From a, from a testing standpoint is that moon rocks have the |
|
| 51:55 | Moon assaults assaults have the simplest geologic . You could ever imagine. First |
|
| 52:01 | all the volcanic rocks, we've already how simple that is. And then |
|
| 52:05 | makes moon moon assaults better than earth ? In terms of simplicity? What |
|
| 52:13 | happened? What might have happened to Earth castle that never happened to |
|
| 52:16 | Lunar castle, yep. It doesn't on the moon. Rain can be |
|
| 52:29 | water. It can be bad for geochemical system. No rain. What |
|
| 52:34 | doesn't happen on the moon that happens on earth? Well, there's |
|
| 52:38 | there's less of it, but gravity is not a geochemical problem. |
|
| 52:49 | , that would sort of fall under same idea of water that, you |
|
| 52:53 | , there's some sort of degradation Um, What kind of changes? |
|
| 53:06 | already talked about. Water changes. , I am thinking about another kind |
|
| 53:09 | change. What happens on what happens the earth all the time? It |
|
| 53:13 | happens on the move, earthquakes, are a consequence, broadly speaking |
|
| 53:21 | there's no tectonics on the moon. is this good? Nothing's happened. |
|
| 53:28 | never been buried. They've never been . More foes. They've never been |
|
| 53:32 | under other things and they've never been on. They are the simplest |
|
| 53:36 | we can imagine furthermore, they're very . So they're unlikely to have been |
|
| 53:43 | in any way and they're very And that's good because we've got this |
|
| 53:49 | of what these half lives are and can then apply them to these moon |
|
| 53:54 | and the if we're wrong about you know, So the thing about |
|
| 53:58 | moon rock, a volcanic rock is matter how you date it, you |
|
| 54:01 | to get the same answer because the temperature of the system doesn't matter because |
|
| 54:07 | were, you know, whether you're , we haven't got into this very |
|
| 54:10 | . But I mentioned earlier that we date when we date something we're talking |
|
| 54:15 | . Not the time of formation at time it passed through a temperature |
|
| 54:22 | but for a volcanic rock, that's same thing. Right? We don't |
|
| 54:28 | don't have to, we don't have distinguish between the day it was formed |
|
| 54:31 | the day it passed through 400°. It's same day or at least it's the |
|
| 54:37 | couple of months, right. I if you have a very thick volcanic |
|
| 54:41 | , it might stay warm for a . Did you see the videos from |
|
| 54:44 | assaults in Iceland last month, There a big eruption in Iceland last |
|
| 54:50 | Go check it out on YouTube assaults . Really good stuff. And you |
|
| 54:54 | see those assaults were flowing down and didn't, they didn't cool off in |
|
| 54:58 | minutes. But I mean, I they're pretty cool now three or four |
|
| 55:01 | later from our geologic perspective, it's , right? So It doesn't matter |
|
| 55:08 | you date a system that tells you when the rock was at 400° or |
|
| 55:13 | or 100°, they all attain that temperature the same time. That's what's nice |
|
| 55:19 | volcanic rocks. Plutonic rocks not the , right? Plutonic rock, metamorphic |
|
| 55:25 | might cool very slowly. And if and this goes back to, you |
|
| 55:29 | , your, your marriage license and graduation from high school, they don't |
|
| 55:34 | to be the same date. But because it takes a long time between |
|
| 55:38 | things. But volcanic rocks don't take long time. So we've got these |
|
| 55:43 | rocks and we've dated them sometimes by to 10 different systems and they give |
|
| 55:48 | same answer. And that suggests that have got the system the decay |
|
| 55:55 | right? And it hasn't changed because we've gotten it wrong in any |
|
| 55:59 | the longer the time we have, more likely we're going to see the |
|
| 56:03 | between these two systems. If I to say you to walk at exactly |
|
| 56:07 | same speed, now go start I probably might not be able to |
|
| 56:12 | by the time you get to the of the room. But if |
|
| 56:15 | if I just let you walk to , I'll eventually see one of you |
|
| 56:18 | a little faster than the other one out. I was wrong. They |
|
| 56:21 | have the same speed. Eventually. know, the longer we let |
|
| 56:25 | So these rocks are as old as got and there's not a problem. |
|
| 56:32 | the decay constants are constant over So here's what we think happened |
|
| 56:40 | If you were if you were to the rate of decay or more more |
|
| 56:44 | , proportionate the probability of decay for different isotopes, the graph of each |
|
| 56:50 | of them is a straight line. never change. And we know |
|
| 56:54 | but this didn't happen that if they buried, they didn't very independent. |
|
| 57:00 | know, I suppose there's some conceivable that somehow the whole isotopes, all |
|
| 57:06 | isotopes are somehow talking to each other slowing down and speeding up in |
|
| 57:11 | Yes, mathematically that's possible. But not possible for them to have changed |
|
| 57:15 | some other way. Although if they if they had all done something like |
|
| 57:22 | , there would be some volcanic rocks would match up in some that wouldn't |
|
| 57:27 | we don't have this problem for you know, we get the same |
|
| 57:31 | whether we look at lunar dissolves or resolves or or division dissolves. |
|
| 57:39 | um you know, there were a of, There were a lot of |
|
| 57:45 | for the age of the Earth, know from the Bible and from the |
|
| 57:48 | of water assault in the oceans. one of the most favorite ones was |
|
| 57:54 | familiar with Lord Kelvin's approach. He Kelvin was this guy as a British |
|
| 58:00 | in the 1850s, wrote this paper series of papers in which he figured |
|
| 58:06 | Earth was a certain size. We how the radius of the earth |
|
| 58:10 | Um and he assumed, he well let's just say that the earth |
|
| 58:13 | out at a certain temperature. He the temperature of molten iron. That's |
|
| 58:17 | really why he was wrong. Um a big hot temperature and then he |
|
| 58:24 | to know what the geothermal gradient So we sent some people down to |
|
| 58:27 | bottom of a coal mine in which was in 1850 was kilometer |
|
| 58:35 | Always kind of impressed me that they 1000 m hole in the 1850s, |
|
| 58:40 | apparently they did, they measured the of the rocks down there, they're |
|
| 58:44 | down there. So that gave him sense of what the geothermal gradient |
|
| 58:48 | even if it was only on the outside. And then he did some |
|
| 58:52 | and he said, Well, a like that starting out uniformly molten iron |
|
| 58:56 | now this big and now it's got outside gradient, that would have |
|
| 59:00 | that would have taken about 30-100 million to do that. And of course |
|
| 59:06 | was a big deal because in this was 1860, which was just |
|
| 59:10 | year after Darwin had published on the of species, in which he was |
|
| 59:15 | about all of this evolution stuff, are very keen on this and said |
|
| 59:21 | that makes a lot of sense. they said, this probably doesn't happen |
|
| 59:24 | . This implies the earth is very . This came on the heels of |
|
| 59:29 | like Hutton and Lyell in the late , 18th and 19th, middle 19th |
|
| 59:35 | saying, you know, the President the key to the past uniformity, |
|
| 59:38 | ism, everything's nice and slow. so, you know, Hutton was |
|
| 59:42 | for when he was asked, how is the earth? He said, |
|
| 59:45 | , I don't know. But there's vestige of a beginning and no prospect |
|
| 59:48 | an end. That's not a But it's a poetic way of saying |
|
| 59:53 | , really old. Right? And Calvin comes along and says it's 30 |
|
| 59:58 | . This bothered many of the geologists a biologist. They thought it was |
|
| 60:02 | small. But the problem was, that he didn't know about radio activity |
|
| 60:09 | radio activity, as I showed you those other equations, It goes this |
|
| 60:13 | to this plus the neutron or plus positron plus energy. There's energy at |
|
| 60:17 | end of that. And that heats the Earth. And so kelvin was |
|
| 60:21 | , not because he picked the wrong temperature, but that he didn't know |
|
| 60:25 | there was heat generated inside the And now we can, you |
|
| 60:29 | we can go back and fix kelvin's but we can also just date a |
|
| 60:33 | straight away because we've got radio The oldest rocks we know about are |
|
| 60:41 | Canada. There's a rock there, about 4.1 should say 4.1 now. |
|
| 60:47 | there are rocks, there's a rock Western Australia. It's a Sandstone whose |
|
| 60:53 | grains include some zircons which have been to 4.4 billion years. That's the |
|
| 61:00 | piece of geologic material we've ever put fingers on. But we still say |
|
| 61:05 | earth is older than that because you , it's not likely we're going to |
|
| 61:10 | the oldest piece because again, Earth this plate tectonics and rain problem. |
|
| 61:18 | sticking around for four points anyway. the moon and the and the meteorites |
|
| 61:23 | us an age of 4.6 billion. So that's the mathematics of dating |
|
| 61:34 | We measure parents and daughters in the the in the lab, we have |
|
| 61:41 | , we haven't gotten into the problem how we get rid of the initial |
|
| 61:46 | talk about that soon. Uh but we if we do that we can |
|
| 61:52 | these numbers on things, these numbers better and better when we find the |
|
| 61:56 | out front, right if you can and I went to a G. |
|
| 62:00 | . A. Meeting maybe 10, years ago now and it was all |
|
| 62:05 | this number here And they were I they were trying to say this is |
|
| 62:09 | away, I don't know what textbook came from. I think when I |
|
| 62:13 | to the GSM anymore recently they were that you know they had gotten to |
|
| 62:17 | point where they were saying that 211.5 211.5 and somebody said well 209.5 you |
|
| 62:27 | , they're pushing the talent And that's of two things, the better machines |
|
| 62:33 | we get, you know, 50 ago, you know, plus or |
|
| 62:37 | 10 million years was a great People were satisfied. Oh thank |
|
| 62:41 | thank you. I learned so much . You're a great guy Nowadays that |
|
| 62:46 | rock, if you reported plus or 10 million years, you know, |
|
| 62:51 | in the back of the room would , what's wrong with these people? |
|
| 62:54 | terrible literature plus or -10 million. Now I should say actually if it |
|
| 63:02 | four billion plus or minus 10 million would be fine. But if it |
|
| 63:06 | 100 million plus or minus 10 that's a real failure this day because |
|
| 63:11 | have moved from where And so the really is not Absolute uncertainty but relative |
|
| 63:18 | , it used to be that 5% fine and now it's a 10th of |
|
| 63:24 | is expected. So that means if have 100 million year old rock, |
|
| 63:30 | of a million years? Should be good uncertain. I mean that's that's |
|
| 63:35 | that's that's good. If you only plus or minus 1000.5 people wouldn't sort |
|
| 63:39 | you know leave the room thinking what terrible lab. But if you started |
|
| 63:43 | about plus or minus three plus minus you might want to explain what it |
|
| 63:48 | that caused your result to be not good. Um So as you find |
|
| 63:54 | better outcrops and then you take them better and better machines. Um The |
|
| 64:02 | spectrometers have improved in the same way your phone or your computer has you |
|
| 64:06 | 40 years ago the ability and it's about in these things. It's about |
|
| 64:11 | , signal to noise. You know you want to have a nice quiet |
|
| 64:14 | electronic signal so that you can measure that tiny little difference between zero and |
|
| 64:20 | you have here. But if you're you're if you're zero looks like this |
|
| 64:24 | gonna miss a lot of stuff. anyway this gets better all the |
|
| 64:29 | And we talked about that talked about um Time is it to 11. |
|
| 64:39 | we generally go here in the afternoon 4 35. Is that correct? |
|
| 64:45 | And then tomorrow it'll be 8:30. . Um Let's take let's take it's |
|
| 64:57 | 12. Let's come back at 2 . Give me a chance to not |
|
| 65:04 | for a minute or two? Should just keep this? What about this |
|
| 65:08 | thing? Should I stop it? I start over? Alright, so |
|
| 65:17 | just be a 10 minutes and eight gap here. All right. |
|
| 65:40 | Yeah, I forgot about 15. . Thank you. More. Very |
|
| 65:50 | . This is what I think that asked them. I had a little |
|
| 65:59 | so I had to say yes. . Oh. Mhm. Yes, |
|
| 69:54 | . Thank you. Got to move another table. But you're running out |
|
| 73:25 | power. Is that gonna reach You're gonna sit here. Mhm. |
|
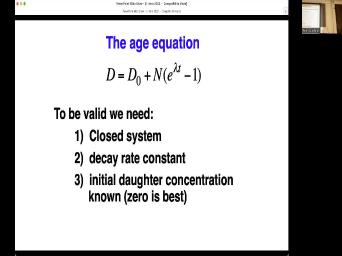
| 73:44 | . So, we've got ourselves an equation now. However, there are |
|
| 73:49 | issues. We've talked about one of already. The decay rate needs to |
|
| 73:53 | constant. We're happy with that. We also um I need to have |
|
| 74:01 | know what the initial daughter concentration Zero would be nice. But what |
|
| 74:06 | we could just do it some other ? Um We'll talk about that coming |
|
| 74:12 | a while, but right now we're talk about this closed system. |
|
| 74:16 | closed system means that we have no or gain of parents or daughters. |
|
| 74:23 | except for the change of parents, daughters within our system. So in |
|
| 74:30 | words, if we find a piece some some well, no, I'll |
|
| 74:33 | say it that way. Nothing comes . Nothing comes out. We can |
|
| 74:37 | internal changes. Parents change too dot close. Uh But the system the |
|
| 74:44 | we have to worry about most often an open system in which we have |
|
| 74:48 | of daughters out of the system. generally this is because of chemical diffusion |
|
| 74:54 | is a thermally activated process. The things are, the more likely you |
|
| 74:59 | to basically leave the system. And this is why uh this is all |
|
| 75:07 | chronology is that we're dealing with the and the temperature. And given the |
|
| 75:13 | , we have to worry about when got to this special temperature, it's |
|
| 75:17 | for different systems. Um so that's we talk about geo chronology. You |
|
| 75:24 | , we have to ask, well are we really doing? Well, |
|
| 75:27 | is this temperature thing that really gets to us. And so we have |
|
| 75:31 | worry about this business of diffusion and is thermally activated process. That means |
|
| 75:38 | goes faster the hotter it is. it's also randomly directed and it's randomly |
|
| 75:44 | . As as shown here, when put this blue dye into this |
|
| 75:48 | it eventually moves out. Now that eventually all the water becomes the same |
|
| 75:53 | . But that's not, that's not the dye knows to seek out the |
|
| 75:58 | , you know, it's just random will even this out eventually. Um |
|
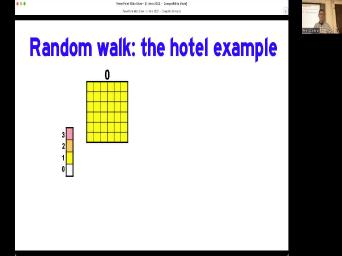
| 76:04 | I've got a very crude example of to think about this with this silly |
|
| 76:12 | x 6 matrix here which I made , you didn't, I did this |
|
| 76:18 | years ago using the sophisticated modeling software as Excel. Um and so you |
|
| 76:25 | 36 elements here. And we're gonna gonna put them in this thing I |
|
| 76:30 | the hotel. This is a very hotel that doesn't have any hallways and |
|
| 76:34 | has, but it has doorways between these rooms. And one more thing |
|
| 76:39 | the doorways is, all the doorways the outside of the hotel are one |
|
| 76:43 | doorways, you can't open them when get back out. So once you |
|
| 76:46 | the hotel, you may not but you can move between rooms |
|
| 76:50 | And this is analogous to geochemical diffusion a mineral. Like, say if |
|
| 76:54 | talking about argon in a biocyte, can move around. But once it |
|
| 76:58 | it outside the mineral, it's much to go away than to come back |
|
| 77:03 | . And so this scale bar on other side here shows the density of |
|
| 77:09 | of the hotel. We're going to that we have one in each room |
|
| 77:12 | the hotel. And then we're gonna through time steps in which we randomly |
|
| 77:16 | one of four directions. So let's do that. And here's an |
|
| 77:20 | after one time step. See some the rooms are empty, some of |
|
| 77:24 | rooms have two people, most of empty rooms are on the edge of |
|
| 77:29 | of the hotel, but there's some ones in the middle. Let's do |
|
| 77:32 | again. We're getting to see more a pattern where there's an emptiness on |
|
| 77:37 | outside, we've even got one room got three in it now, but |
|
| 77:41 | deal. Um after three time four, five time steps, we're |
|
| 77:48 | to see. And if we were there is a pattern and of course |
|
| 77:51 | is silly because we're only doing five steps in a six by six |
|
| 77:56 | If I if I bothered to do in 1000 by 1000 system, it |
|
| 77:59 | look prettier, but I didn't do . Somebody ought to write a, |
|
| 78:04 | know, python code to do this than this. But I did this |
|
| 78:08 | years ago, before that existed, can say that the initial concentration was |
|
| 78:13 | and then it moved to 26, , 25 down to 21. This |
|
| 78:19 | just randomly moving, you know, directions. This is an okay analogy |
|
| 78:27 | what we're talking about, We're just around and over time we will set |
|
| 78:30 | a gradient in which there is less on the edge. Now, the |
|
| 78:35 | activated part is all of this happens , but if you turn up the |
|
| 78:39 | , you know, maybe we say the time this thing, this is |
|
| 78:42 | day between if the temperature was hotter one hour or something, you |
|
| 78:46 | all of this would happen faster. that's the temperature association. And so |
|
| 78:52 | that silly example to begin, let's a more sort of abstract situation and |
|
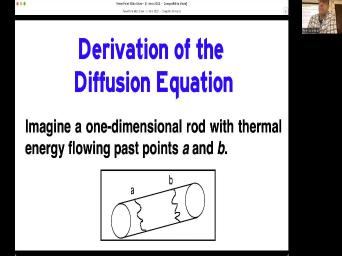
| 79:00 | going to imagine a one dimensional if you will and we're only going |
|
| 79:05 | consider activity along that one dimension. we're going to say that there is |
|
| 79:11 | thermal energy passing through two points on Rod points A&B. And by the |
|
| 79:16 | this this this this um this derivation I'm about to go through, we're |
|
| 79:21 | it's for thermal energy heat. But mathematics and all of the assumptions are |
|
| 79:27 | the same. If we talk about , Argon NFL's bar sugar in your |
|
| 79:35 | concentration of mass and something same Just a little easier to think of |
|
| 79:40 | in terms of heat right now, will eventually uh generalize it to any |
|
| 79:46 | those things. So we've got heat past A and B. We can |
|
| 79:52 | that a little more formally to say the total heat energy between A and |
|
| 79:56 | would then be the integral between A B. Of this function energy which |
|
| 80:01 | a function of distance and time. just making things look Mathy right |
|
| 80:08 | Okay, um if we say that we can relate the change in |
|
| 80:16 | energy in time to the flux of rod. Where ask, is the |
|
| 80:21 | of energy coming in at A and . Is the amount coming out of |
|
| 80:27 | . And subtract those two and we whatever generation is going on inside, |
|
| 80:33 | know that's something that kelvin did not , but if we we take the |
|
| 80:37 | and we take the internal heat that's just another way of writing that |
|
| 80:41 | over there. Um Okay, so good is that? Well we can |
|
| 80:49 | equate those two things and rearrange and get this that the partial differential of |
|
| 80:56 | respect this is plus the blocks with this minus whatever we have here, |
|
| 81:03 | of all that is equal to But good news is this is only |
|
| 81:08 | if the inter grant itself is equal zero. So when you get rid |
|
| 81:12 | all that stuff and just say that of these people here and that becomes |
|
| 81:16 | so now we have an expression for distance flux and heat generation. Well |
|
| 81:24 | usually describe materials by their temperature, by their thermal diffuse acidity. So |
|
| 81:29 | want to convert a few things we to know, it's the specific heat |
|
| 81:32 | the material, the mass density of material. And for the moment we're |
|
| 81:36 | to say that those are also functions X. You know, somehow this |
|
| 81:40 | varies along here, we can we we can get rid of that assumption |
|
| 81:45 | . But for now let's say it's a function of location. And so |
|
| 81:49 | can then change this energy into the times the heat capacity times against |
|
| 81:55 | So now we have this equation that's in terms of temperature flux of these |
|
| 82:04 | properties. So that's maybe a little thing to think about now then, |
|
| 82:11 | think about what what what what what concerns we can add to our |
|
| 82:17 | , we know for example that if constant, there's no transfer of thermal |
|
| 82:22 | and we know that if there are differences, the heat energy flows from |
|
| 82:26 | to cooler and the greater the temperature , the greater the heat flow. |
|
| 82:32 | is why you have to be careful picking up a pan that's hot because |
|
| 82:35 | will transfer that heat straight to your because of the delta T. It's |
|
| 82:39 | big. You can't burn yourself if delta T is small, even though |
|
| 82:43 | more energy in that thing. And finally the flow of heat energy will |
|
| 82:47 | for different materials, even when the T is the same. So, |
|
| 82:51 | are all things we know about we can write them mathematically like this |
|
| 82:56 | say that flux is equal to the conductivity, times the rate of times |
|
| 83:05 | the gradient of temperature with distance. equation said, is a mathematical way |
|
| 83:10 | putting all four of those things we said into one equation and K K |
|
| 83:17 | X is thermal conductivity in question. would have units of joules per |
|
| 83:21 | per centimeter per kelp. Alright, good is that? Well, because |
|
| 83:28 | have we had that was an equation that. We're gonna substitute that in |
|
| 83:33 | . Put that in here now, were getting now to see what we've |
|
| 83:37 | here now, we have just an that is in terms of these material |
|
| 83:41 | . They may not go see and left here is we have temperature, |
|
| 83:48 | and distance. We've simplified it just those basic things. If we assume |
|
| 83:54 | the specific heat, the density and thermal conductivity are all constants that we're |
|
| 83:58 | dealing with one kind of material, or copper wire or felt bar. |
|
| 84:04 | can say it's not a function of , that's a constant. We can |
|
| 84:08 | all of those constants up together, can get rid of the constant, |
|
| 84:12 | rid of this thing. And now they're all constants, we can group |
|
| 84:16 | all together into one constant. So we have that the partial differential of |
|
| 84:22 | with respect to time is equal to constant D which we called the facility |
|
| 84:28 | the second partial differential with temperature with to distance. And there was some |
|
| 84:36 | was some differential equation stuff in but don't worry too much about |
|
| 84:40 | Um this is told fixed 2nd law the diffusion equation. And this is |
|
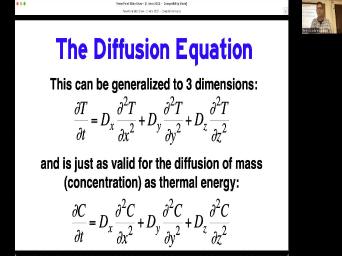
| 84:45 | be fundamental in our ability to understand temperature and time are related in these |
|
| 84:52 | topic systems. Um and I and and this this can be generalized |
|
| 85:00 | three dimensions if in fact the it is not the same in all |
|
| 85:05 | . And why wouldn't it be? , let me let me let's skip |
|
| 85:18 | here and maybe answer better here. can also it's just as valid for |
|
| 85:22 | diffusion of mass that is for thermal . So we'll change from temperature to |
|
| 85:28 | but we can then have a diffuse in the X. Direction, |
|
| 85:31 | Direction, the direction. Why might want to do that? The diffusion |
|
| 85:35 | some mass? Why would we why it be better to say that the |
|
| 85:41 | this direction is different from this Well, you're thinking about strategic graffiti |
|
| 85:54 | we really need to be down in right now. But you're thinking the |
|
| 85:57 | you've got right but use that idea put it inside a mineral. You |
|
| 86:08 | describing a reservoir which isn't the same . Our minerals always the same everywhere |
|
| 86:16 | have. I mean the C. might be different than the A. |
|
| 86:19 | , Right? That's what this going . It's not always the case that |
|
| 86:22 | should say that the diffusion of let's say, you know, we |
|
| 86:27 | to buy a tape strong, you , layering in one direction, not |
|
| 86:33 | the other direction. So we won't that the diffuse city going across the |
|
| 86:38 | is the same as parallel to the . So we may have to we |
|
| 86:42 | have to modify this simple equation by that we can't say that there's a |
|
| 86:47 | , it decisively depends on direction. that ends up giving you giving you |
|
| 86:53 | but it's still a fairly generic sort thing where we're talking about concentration and |
|
| 86:59 | and distance. But um couple of yet to do that will help us |
|
| 87:06 | . The first thing is is that D. A. In these |
|
| 87:09 | Either this simple equation or this slightly complicated equation. The D. Is |
|
| 87:15 | as a number. But that's But important thing note and this is where |
|
| 87:20 | diff the thermal relationships really get important that diffuse entity is temperature dependent. |
|
| 87:28 | talked about this and you know you diffuse your sugar into your tea faster |
|
| 87:33 | it's hot, tea is cold. And so mathematically we can say that |
|
| 87:38 | diffuse acidity of something is dependent on . In this sense is that there's |
|
| 87:44 | some some diffuse city do not. we can mathematically we can think of |
|
| 87:49 | the diffuse city at infinite temperature times . To the minus E. Over |
|
| 87:56 | . T. Where E. Is activation energy of the process. And |
|
| 88:02 | energy is a barrier that reactant must . For reaction to proceed. You |
|
| 88:06 | to put energy into something. It's if you you know there's an activation |
|
| 88:10 | for this pen to get to the . Once I put a little bit |
|
| 88:14 | energy into the system then then other take over. But that first bit |
|
| 88:19 | to be done. And so that's of what you can think, activation |
|
| 88:22 | is and um are is just a . The ideal gas constant. And |
|
| 88:30 | the gas constant should be in there kind of funky but we won't worry |
|
| 88:33 | that. And either just use the number 1.987 if you're using calories 8.317 |
|
| 88:39 | you're using jewels and then T. temperature T. Is in kelvin's. |
|
| 88:46 | important thing to meet in mind is we're doing any of these calculations, |
|
| 88:49 | have to use the temperature scale, Celsius won't do certainly Fahrenheit won't do |
|
| 88:56 | only. Okay, so what we then is an equation that says that |
|
| 89:01 | the different city is dependent on This e. Is another one of |
|
| 89:05 | material properties. The activation energy of argon through a feldspar is sub |
|
| 89:11 | the activation energy of moving led through zircon is a bigger number. Um |
|
| 89:17 | then ours are constant. And Then affects this whole thing. |
|
| 89:22 | What D not is you know the that will learn how to figure |
|
| 89:27 | But but no matter what number this , you can see how what this |
|
| 89:32 | up being is strongly dependent on temperature it's in the exponent. And so |
|
| 89:39 | at the math and tell me what to V. When T gets |
|
| 89:54 | the negative exponents. But what the skis down here. So they |
|
| 90:04 | the negative and this cancel each other , This gets bigger. What happens |
|
| 90:09 | ? Let's get bigger. What happens this? No, no it gets |
|
| 90:21 | minus C. Over R. With this number is bigger. This |
|
| 90:28 | thing is smaller but it's a So then this this this quantity gets |
|
| 90:35 | with this quantity when this thing gets . So it and let me I |
|
| 90:41 | I have some examples here. Here's example. These are these are sort |
|
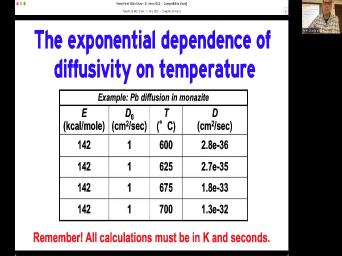
| 90:46 | typical values for some real real This is the example of diffusion of |
|
| 90:52 | in a felled spot. These are are real numbers, you might use |
|
| 90:56 | the the activation energy and I'm using . Some other people who use |
|
| 91:02 | And I'm actually using kilocalories. So you were to type this into the |
|
| 91:06 | , you wouldn't put in 29, put in 29,000. So those 29 |
|
| 91:12 | de not is five. So we calculate, you know, just going |
|
| 91:16 | to this equation and with the right we can push this in and we |
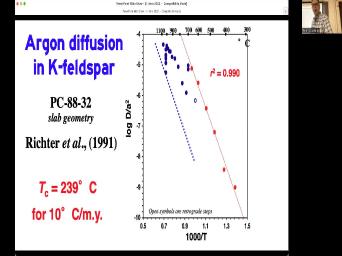
|
| 91:20 | say that if he is 29 kilocalories did not is five. If we |
|
| 91:26 | 450 degrees, but not using 100 50 you have to change that to |
|
| 91:32 | , calculate the diff utility of that would be five incentivized 15 centimeters square |
|
| 91:38 | . That doesn't mean very much to right now. But just just look |
|
| 91:41 | happens when you increase this value by 50°. Go through the same calculations |
|
| 91:47 | we get two times center mass That's a factor of almost 100. |
|
| 91:54 | a factor of what 80 going from to here. We've we've we've increased |
|
| 91:59 | diversity of the system by a factor 80 in just 50° change. What |
|
| 92:06 | when we do 100 degrees change, get up to 250 degrees. We've |
|
| 92:09 | 3.8 times 10 to minus 12. almost 1000 fold change In the D |
|
| 92:15 | by changing the temperature by 100°. And not even getting hot yet. That's |
|
| 92:21 | only 250°. Um That's for argon in Celts bar temperatures we can keep |
|
| 92:28 | let's go to go to 350 Now we're at 67, 10 to |
|
| 92:33 | to minus 11. Go back when were when we were at 100 we |
|
| 92:37 | from 150 to 350. That's 200 . Look what were here, we |
|
| 92:40 | at 515 Six times 10 months That's a factor of 10,000. And |
|
| 92:47 | we did was go up 200°. and this is just one system, |
|
| 92:53 | are others. But here's another This is an example of where we |
|
| 92:58 | uh the diffusion of lead in You'll notice that the activation energy here |
|
| 93:02 | much higher. That tells us that much more difficult to get this system |
|
| 93:08 | . And that's because we're talking about before it was our guy. Why |
|
| 93:13 | it harder to move led around. me. No, our argon argon |
|
| 93:20 | a nerd. But it's easier to than than lead. This is |
|
| 93:29 | Right here. We're just talking about mass. Uh We will we will |
|
| 93:34 | the inner qualities of argon that's important other things. But for now it's |
|
| 93:37 | we're talking about moving it once it's lead is Uh five times bigger. |
|
| 93:44 | so the activation enter is big. and so that's why we that's why |
|
| 93:49 | even though we move up to a much hotter than we were before. |
|
| 93:53 | we're at 600° but the defensive it still hugely lower because that number is |
|
| 93:58 | hot. But so that's one thing that with that number high, this |
|
| 94:03 | comes really small. But look here we go from 600 degrees 700 |
|
| 94:10 | we go from three times 10 to 36 to 177 minus 32. |
|
| 94:15 | a factor of about 600 here. ? Just 100 degrees. So that's |
|
| 94:21 | an illustration of how important temperature is these geochemical concerns and you know that's |
|
| 94:29 | we don't just measure the age of mineral we age. We measure the |
|
| 94:33 | in which this thing, this diffuse slows down sufficiently in the minimum and |
|
| 94:38 | dependent on these things like E and . Nod. And so I'll explain |
|
| 94:45 | how we learn what those values But this just to illustrate how very |
|
| 94:50 | temperature is because it's in the expo concept of exponential changes why we can |
|
| 94:56 | a metamorphic rock in our hands, rocks that you know you put some |
|
| 95:01 | and some quartz together and sit on on the table here. Just sits |
|
| 95:05 | clay and quartz. Right? But you bury it down at 400 degrees |
|
| 95:09 | 10 kg bars for 20 million it's not clay in courts anymore, |
|
| 95:14 | shift. But that shift comes back surface and you put it on the |
|
| 95:18 | . Why isn't it turning back into in courts? It wasn't it didn't |
|
| 95:22 | to be garnet and muscovite here on stage. Why won't it turn |
|
| 95:30 | Well it will it's just doing it slow. You need a couple of |
|
| 95:38 | years for this to happen because we talking about the difference in diffuse acidity |
|
| 95:43 | 500° and 20°. And you can see that might be a factor of in |
|
| 95:50 | case of these metamorphic reactions, it's factor of several billion. And so |
|
| 95:55 | takes 20 million years to do at will take, you know, 100 |
|
| 96:02 | times longer at the surface of the . It's not that that that that |
|
| 96:07 | is stable. It's unstable and it'll turning into clay one of these days |
|
| 96:15 | of years exponential dependence is what's going . So taking us back to this |
|
| 96:22 | where where we just talked about t a number but it's actually this exponential |
|
| 96:27 | inside. We can um mathematicians help out by pointing out that we can |
|
| 96:35 | can use this equation very um profitably we make certain assumptions about geometry, |
|
| 96:44 | if we if we assume that the we're looking at is a sphere or |
|
| 96:50 | cube or maybe an infinite slab or infinite cylinder, then these provide us |
|
| 96:56 | um boundary conditions that allow us to the equation. You're nodding from your |
|
| 97:04 | , you know, that's the Right? So this gives us a |
|
| 97:07 | to turn this generalized equation into something useful. And I won't go into |
|
| 97:13 | derivations. That's a that's a bunch partial differential equations stuff. But if |
|
| 97:18 | make those assumptions, you can end with this and don't worry, I |
|
| 97:21 | these numbers can't read them here, just jump jump to this. So |
|
| 97:24 | are four solutions. The diffusion If you choose sphere or cylinder or |
|
| 97:30 | or Q. Let's just look at sphere. So we can read the |
|
| 97:34 | . And what we have done here we've introduced before we had before we |
|
| 97:40 | temperature time distance and diffuse city when do this, we ended up with |
|
| 97:47 | up with uh what do we got ? We've got uh time diffuse |
|
| 97:55 | Now diffuse city is a function of . And then the other thing we |
|
| 98:00 | then there's these are a bunch and . Here's a A. Is the |
|
| 98:05 | . Is either the radius of the , the thickness of the slab, |
|
| 98:10 | side of the cube or the radius the cylinder. That's a dimension that |
|
| 98:14 | get out of these assumptions. So we have that's what A. Is |
|
| 98:19 | . Is what it was before. is time and temperature is inside of |
|
| 98:26 | . And then one more thing is . F. Is the uh the |
|
| 98:31 | of diffusion that is lost. If have a certain amount of thing and |
|
| 98:38 | subject it to some new conditions and , we say we lose 10% of |
|
| 98:44 | . F would be .1. And we have to learn f through our |
|
| 98:50 | , we start out with some and we do an experiment and we |
|
| 98:53 | we get this much, sometimes we know the exact value of f until |
|
| 98:57 | have the whole experiment done. So we can because it has to be |
|
| 99:00 | relative number, it's not it's not a relative to the total. So |
|
| 99:05 | we can't figure out what f is we're done. But eventually we know |
|
| 99:08 | that represents 10% and that's 12% and forth. So now we can solve |
|
| 99:14 | equation and learn some of these things were interested in if we know what |
|
| 99:18 | . Was. And it is the that these differential equations things either give |
|
| 99:23 | this this beautiful equation here. That's all f no matter what. But |
|
| 99:29 | problem with the equation for all You see, it's it's an infinite |
|
| 99:33 | And it's not one of those infinite that converges really rapidly. Some, |
|
| 99:37 | know, it may sometimes depending on level of concern, you may want |
|
| 99:41 | run this, you know, 10,000 , which I suppose nowadays isn't all |
|
| 99:47 | computational e expensive. But We can by without doing any of those 10,000 |
|
| 99:54 | . If we just take these two here, which are approximations, then |
|
| 99:58 | involve an infinite series. And we use this one as that between 1.85 |
|
| 100:03 | this one between .85 and zero. so take a look at this |
|
| 100:09 | For example, we now know we say that F is approximately equal to |
|
| 100:13 | over pi to three halves times square pi square DT on a squared minus |
|
| 100:18 | D t n a square. That's thing. We'll use in a |
|
| 100:23 | So now we have an equation that , that can, we can use |
|
| 100:27 | our experimental data. So we're going then use that to get to this |
|
| 100:35 | called closure temperature, which I may hinted at but not really defined just |
|
| 100:43 | . The geo chronological closure temperature of system is the temperature at which the |
|
| 100:50 | and loss of a particular species are . Let's say potassium is decane to |
|
| 100:55 | car. If we are at our temperature for this system, the rate |
|
| 101:00 | which that argon is leaving the system of this thermal diffusion is equal to |
|
| 101:05 | rate at which is being born because radio activity. So we have a |
|
| 101:10 | have some argon in the system, not very much because most of its |
|
| 101:14 | as fast as it's coming. Um way to think about this is that |
|
| 101:22 | closure temperature of the Chronicle. The temperature is the temperature of this chronological |
|
| 101:29 | at the time represented by its parent . That means if we data bio |
|
| 101:34 | , we get 100 million years that bio type was that its closure |
|
| 101:39 | 100 million years ago. Now that doesn't tell us what that closure temperature |
|
| 101:43 | . But theoretically that conceptually we are the closure temperature when at the time |
|
| 101:49 | the age we got whatever that age . Um I should point out that |
|
| 101:54 | concept is not, is not restricted chronological systems. Uh, metamorphic tetralogy |
|
| 102:00 | paleo magnetism use the same thing paleo . The curie temperature temperature, you |
|
| 102:05 | up and you lose magnetism as we'll in in in geo chronology, it's |
|
| 102:10 | like turning on a light switch. not this perfect on off deal just |
|
| 102:14 | for magnetism. If you get close the curie temperature, the magnetism will |
|
| 102:18 | down and then eventually the go So from our perspective, let's look |
|
| 102:26 | this diagram, we've got a situation we're gonna talk about cooling here, |
|
| 102:30 | which we have temperature and time, is on the X axis in both |
|
| 102:34 | these, on this one, we temperature on this one, we have |
|
| 102:37 | ratio of daughters to parents. And we're cooling we're hot at some |
|
| 102:42 | high temperature here above the closure we'll be at a temperature sufficiently hot |
|
| 102:47 | that the daughter dependent ratio is equal zero. That's because the daughters are |
|
| 102:52 | the system very quickly, no matter many we produce, they just go |
|
| 102:58 | . But as we lower the we get closer and closer to the |
|
| 103:02 | temperature, we begin to retain a of them. Maybe not, you |
|
| 103:09 | , we're not, we're not some of equilibrium because we're still losing a |
|
| 103:12 | of them. But we're, the patient ratio is rising up a little |
|
| 103:17 | and it keeps rising and we pass the closure temperature and we get to |
|
| 103:22 | little bit below the closure temperature and we get to a temperature like here |
|
| 103:26 | which effectively not truly but effectively, retain all of our daughter products. |
|
| 103:33 | , now again, I said, truly in the same way as |
|
| 103:37 | as the shift is not truly happy on earth surface, but it's pretty |
|
| 103:44 | . Okay, effectively, we have to a point in which all of |
|
| 103:48 | products are retained. Here's a point none of them are retained. Here's |
|
| 103:54 | point where all of them retained, a kind of middle ground in the |
|
| 103:57 | . Um And if you see this line, this dotted line goes back |
|
| 104:01 | this point here and we read up and over here, that's the closure |
|
| 104:07 | . And this dotted line is important if you were to, if you |
|
| 104:10 | to take the daughters that are produced this side and the daughters produced on |
|
| 104:14 | side, it's the same as if just had a straight line. So |
|
| 104:17 | mathematically as if there was a light turning on and off, but physically |
|
| 104:21 | doesn't happen that way. So we down here, back up here, |
|
| 104:26 | the closure, strictly speaking, we think of it more as a closure |
|
| 104:30 | kind of start here and eventually we through it. And as we'll see |
|
| 104:34 | what this temperature is can depend on things like how fast we go through |
|
| 104:39 | , the faster we go through the higher it is. But I'll |
|
| 104:43 | to that in a sec now. , here's what we're gonna derive then |
|
| 104:50 | formal expression for closure temperature. We're to consider cooling over an interval that |
|
| 104:59 | on our cooling, our closure which is another way of saying the |
|
| 105:03 | of our cooling interval is equal to closure temperature. And we're gonna also |
|
| 105:09 | that over this interval, the diffuse he drops by a factor of E |
|
| 105:14 | of course we're going to do that it's mathematically simple. We can deal |
|
| 105:17 | some logarithms that way. And so can write that to say that the |
|
| 105:22 | city in one case over the other . One divided by T. |
|
| 105:25 | Two is going to be able to not E. To the minus |
|
| 105:29 | Over R. T. One You know? And so forth. |
|
| 105:33 | both of those both of those potions equal to eat. So we can |
|
| 105:41 | um log on both sides. And we have the the the the the |
|
| 105:50 | go away. Right? And so have um and then we can because |
|
| 105:56 | both exponents we can put that inside . So we have this take the |
|
| 106:01 | of both sides. We have one E. R. Times this expression |
|
| 106:06 | in it. Um We're going to a few substitution is here and |
|
| 106:10 | That might not seem obvious why. you'll see that they take us where |
|
| 106:14 | want to go. The first of substitution is this one we're gonna say |
|
| 106:18 | one over T one minus one over two is approximately equal to T. |
|
| 106:23 | minus T. One over the quantity plus one T squared. Why are |
|
| 106:27 | doing that? We want to make substitution. We're gonna note that T |
|
| 106:32 | minus T. One is the same delta T. And W. |
|
| 106:35 | Is the same thing as cooling rate time. That's nice to see why |
|
| 106:41 | good. And we're also gonna note T. One plus T 2/2 squared |
|
| 106:48 | the same thing as the average of interval square. But we've already defined |
|
| 106:52 | average of the interval of this is closure temperature. So by making |
|
| 106:57 | by making that substitution, making that , we can get it in this |
|
| 107:03 | here, which we like a little and we substitute that back into what |
|
| 107:07 | have now. Is that one equals times DT DT Times two times the |
|
| 107:13 | in time times are times the closure square. So now we have an |
|
| 107:19 | that's got closure temperature. We're honing on a good thing here. Um |
|
| 107:25 | we're not done yet for the We're gonna assume spherical geometry and we're |
|
| 107:32 | apply only the first term that we about earlier. We're gonna we're gonna |
|
| 107:35 | that because it really adds very little it. So we're just gonna say |
|
| 107:40 | , we can say that this is case from that equation we had |
|
| 107:45 | but we know that closure temperature, is s equal to At this .5 |
|
| 107:52 | they're balanced. So that's we get of Athens put a number in there |
|
| 107:57 | . And so now we can say 0.5 equals another number. All we |
|
| 108:01 | now is that we can rearrange all and say that delta T. And |
|
| 108:07 | is equal to 1/55 55 comes from those numbers being put together and we |
|
| 108:14 | substitute that back into our equation. now we have this thing, this |
|
| 108:19 | we've got e cooling rate, uh constant closure, temperature, diffuse city |
|
| 108:28 | . Um we're gonna make one more , remembering that that um the here |
|
| 108:34 | had here we have D on a , remember a. Is it is |
|
| 108:37 | dimension of our system, the radius our sphere. But we're going to |
|
| 108:42 | that really D on a squared is is the same concern we had |
|
| 108:48 | We just divide our equation here by squared and we know that DNA squared |
|
| 108:54 | squared. And my C. R. T. Because this gets |
|
| 108:56 | back to not having the exclusivity be number but a function of temperature. |
|
| 109:02 | we we put that in, we've DNA squared down there. We don't |
|
| 109:07 | that. We're gonna substitute that in . We get that thing That now |
|
| 109:12 | done. Except we've changed one thing we had 55 before I put |
|
| 109:18 | A because that's a variable that we change depending on our geometry when we |
|
| 109:23 | we do it. But for a we get 55. If we go |
|
| 109:26 | this whole exercise again, it becomes for a cylinder and 8.7 for a |
|
| 109:32 | . And and we may come to that we decide to use one of |
|
| 109:35 | geometries. We just change that big that's just a that's a model |
|
| 109:41 | But aside from that, let's examine equation and see what use it |
|
| 109:46 | Now the first thing I want you note is something a little funky look |
|
| 109:52 | that. Is that a problem? we done yet? We've not got |
|
| 110:01 | . C. On both sides of equation. And we do we have |
|
| 110:04 | keep going. We can't keep going can we will never get T. |
|
| 110:11 | . On one side of the Why not? Lot? As soon |
|
| 110:15 | we get rid of the live we're have an X. Phone. So |
|
| 110:21 | are we going to deal with Never seen an equation like this |
|
| 110:26 | So that it's not a problem really this can be solved by iteration. |
|
| 110:32 | you have to do is put a in here. It really doesn't matter |
|
| 110:37 | number you put in there. I to chart with 600 but if you'd |
|
| 110:42 | start with six, that's fine. pick a non zero number. Well |
|
| 110:47 | I guess you're squaring it, you take a negative number um you know |
|
| 110:52 | you want and and get a number take the result of that first |
|
| 110:58 | Put it back in, do that times and you'll you'll never you know |
|
| 111:02 | be the same. You know if put in 600 the next number might |
|
| 111:06 | know, depending on all these other . 600 will will will give you |
|
| 111:10 | 25. Put in 3 25 you 3 27. Put in 3 27 |
|
| 111:15 | 3 26 put in 3 26 you 3 26 you're done. So you |
|
| 111:20 | , it's very simple to set up columns in Excel or something like that |
|
| 111:23 | to do it five times. So not a problem. But that is |
|
| 111:28 | , you know, on on first you might say well this isn't done |
|
| 111:31 | we're trying to solve for this. on both sides. Yes. But |
|
| 111:34 | how you deal with that. But that then consider the effects of the |
|
| 111:39 | terms. What happens when we increase energy to close your temperature? |
|
| 112:02 | What did you say? It Can you explain Yeah, when this |
|
| 112:12 | bigger this whole thing gets bigger. means this whole thing gets bigger. |
|
| 112:17 | we've also got an E down But because it's in the bottom, |
|
| 112:21 | this gets bigger this gets smaller, means this whole thing gets bigger. |
|
| 112:26 | activation energy close your temperature goes What about cooling rate? This goes |
|
| 112:44 | . This goes down which means this up. So the rate at which |
|
| 112:51 | cool will actually determine what that special is, the faster you cool, |
|
| 112:58 | higher the temperature, it's actually more to actually turn it around and say |
|
| 113:02 | slower you cool because the effect is pronounced on really slow cooling rates in |
|
| 113:10 | this business. We generally sort of if we have no other reason, |
|
| 113:15 | pick a cooling rate of 10°C That's sort of a standard cooling rate |
|
| 113:21 | granted, let's say. But something's slower than that. And if you |
|
| 113:26 | to find some, some systems that cool there, say it's a metamorphic |
|
| 113:30 | that sits down there at the bottom the crust for a really long |
|
| 113:34 | you might have a cooling rate of of a degree C per million |
|
| 113:37 | The difference between the 10th and 10 be 40 or 50 degrees. Once |
|
| 113:43 | get up above 10 or 15 degrees , the difference between 10 and 1000 |
|
| 113:47 | only be three or four degrees. , What about um, let's |
|
| 113:55 | let's do them individually. What about diffuse city at infinite temperature? If |
|
| 114:02 | if that increases, what happens to system to the closure temperature? It |
|
| 114:11 | . And that should make sense because talking about diffuse city is the ease |
|
| 114:15 | which we're moving through the system, ? The easier it gets to move |
|
| 114:19 | , the faster we should be able get out, the less energy we |
|
| 114:22 | to put in the system. So closure temperature ought to be slow, |
|
| 114:25 | to be low and mathematically, that out here. When we make the |
|
| 114:29 | bigger, close your temperature goes down then finally, what's the effect of |
|
| 114:36 | the diffusion dimension. When the diffusion gets bigger, what happens to the |
|
| 114:48 | ? You've got another denominator to worry here. When this gets bigger, |
|
| 114:54 | gets smaller. Which means this gets , which means this gets bigger. |
|
| 115:01 | should make sense. I mean, know, you shouldn't just be thinking |
|
| 115:04 | the math and think about what the we're talking about here. What |
|
| 115:07 | what is a again, it's the you have to travel and the greater |
|
| 115:12 | distance you have to go. That you ought to, you know, |
|
| 115:15 | get out. It would help to harder. So if you have a |
|
| 115:19 | tiny crystal, you know, a that's like a micron long, it's |
|
| 115:24 | easy to leave that system, the temperature, you have to get really |
|
| 115:28 | in order to keep things from getting of that. The closure temperature of |
|
| 115:31 | teeny little crystal is small. The temperature of the crystal, the size |
|
| 115:35 | this room. Um it's gonna be because it's, you know, it's |
|
| 115:39 | different dimension. Now, it is case that we sometimes only refer to |
|
| 115:43 | thing as this closure here because from , from the data that we |
|
| 115:47 | which I'm about ready to describe to . The data we get. It's |
|
| 115:53 | hard to tell, you know, you measure something with so much argon |
|
| 115:56 | out of your sample or your lead so far in your zircon. Sometimes |
|
| 116:01 | hard to measure the difference. It's to understand the difference between a bunch |
|
| 116:08 | argon that moved slowly through a slowly through a small distance or quickly |
|
| 116:14 | a large distance. They can balance , right? So you can get |
|
| 116:18 | same amount. We had a bunch people in this room and they were |
|
| 116:23 | walking out the door and I just , oh, there's 100 people in |
|
| 116:26 | hallway. You know, was that that the result of one person an |
|
| 116:31 | for 1000 hours? Or was it people a minute for one minute. |
|
| 116:36 | same. The same number of people out in the hallway. So sometimes |
|
| 116:40 | difficult to drop these apart and that's we may go back to these equations |
|
| 116:49 | . They come together. Now time added. But key on a |
|
| 116:53 | They always go together and I'll explain even better. Okay, so, |
|
| 117:05 | . So, yeah, we did . All right. So now we |
|
| 117:08 | to experimentally determine these diffusion parameters because have asserted at the beginning of this |
|
| 117:14 | we have, we know what the temperatures are at different things. How |
|
| 117:18 | heck do we know that comes from ? Let me explain a couple something |
|
| 117:24 | . Um, but before we do , let's just again, do a |
|
| 117:27 | thought experiment about things. We ought know about this for a diffusion |
|
| 117:33 | We need to have a few things happening. The phase must remain stable |
|
| 117:37 | the experiment. we don't want the to melt, for example, because |
|
| 117:41 | we wouldn't be measuring the diffusion in thing and be some other thing. |
|
| 117:46 | don't even want it to change in other ways. Like muscovite was it |
|
| 117:51 | notoriously difficult mineral to understand the closure of argon. And muscovite because muscovite |
|
| 117:57 | a hydro mineral and we have to these minerals. This this organ has |
|
| 118:02 | be measured in a vacuum because we're 1% argon, right? A lot |
|
| 118:07 | argon in the atmosphere compared to the of argon and a little mineral. |
|
| 118:11 | you gotta put the mineral and you pump it down. It's a |
|
| 118:14 | very low background one billion times less inside them, but at a billion |
|
| 118:20 | less pressure. And you turn up heat a little bit, what's going |
|
| 118:23 | happen to the water in that must . It's gonna boil away. But |
|
| 118:28 | soon as you get rid of the from your muscovite, it ain't |
|
| 118:34 | Some non hydro layered silicate and the city that you're measuring the subsequent experiment |
|
| 118:41 | be on that new thing you made on the miserable that exist in |
|
| 118:45 | So we've got to be careful about changing our mineral as we go. |
|
| 118:51 | that's the problem, muscovite, for , what people just guessed at the |
|
| 118:55 | of temperature of muscovite for a very time. The closure temperature of argon |
|
| 119:00 | feldspar. Easy peasy Because there's no in feldspar and we can heat feldspar |
|
| 119:06 | to about 1200° before it melts. we've got a lot of data |
|
| 119:10 | whereas in Muscovite you heat it up about 600° and it falls apart. |
|
| 119:18 | It would be nice if if the diffusion length scale was known, but |
|
| 119:23 | might not know that, but we at least try and get all of |
|
| 119:26 | grains that we measured in our experiment be the same size. We'd like |
|
| 119:31 | imagine that we could use one of solutions solutions of the diffusion equation. |
|
| 119:36 | it make sense to use a sphere a sheet or something like that? |
|
| 119:40 | mean at some level we're gonna have use an abstraction and use one of |
|
| 119:44 | models. Um It'd be better if aggregate was only one size. Of |
|
| 119:50 | we don't want to mix two minerals because there'd be different things going |
|
| 119:55 | And it would be nice if the distribution of the diffusion was known. |
|
| 120:00 | the best known would be uniform across , they're all the same to begin |
|
| 120:06 | . Sometimes we have a good sense that, But and but the 7th |
|
| 120:10 | is a laboratory concern is that you the heat to be ice a |
|
| 120:19 | Um My oven that I have at . It's really bad about heating up |
|
| 120:23 | you know, if I want to something at 400°. I put it at |
|
| 120:27 | and then I come back an hour because it takes a really long time |
|
| 120:31 | get up to 400°. That's okay for a cake if you have time. |
|
| 120:35 | it's terrible for making these experiments because of the diffusion diffusion is still going |
|
| 120:40 | as we're getting up to 400 And you can't just put the cake |
|
| 120:45 | later because you've got, for you've got to have it under |
|
| 120:48 | So you wanna you wanna an apparatus goes hot really quickly and so you |
|
| 120:53 | use a, you know, a kitchen oven for this approach. |
|
| 120:59 | anyway, going back to so so are just sort of concerns for the |
|
| 121:05 | probably could have skipped them. But we get back to our equation for |
|
| 121:10 | festivity, the over a squared equals squared minus ea over rt let's take |
|
| 121:16 | log of both sides of that Why? Because if we do, |
|
| 121:20 | we can look at that as as an equation of a straight line where |
|
| 121:26 | uh why access is logged on a ? The X axis is one |
|
| 121:32 | T The slope of the line is the over are the intercept is the |
|
| 121:37 | of the knot on a square. so there you go. Right. |
|
| 121:44 | , and they and these things in boxes are things we measure in the |
|
| 121:48 | , we can measure the d Not a square because remember we get that |
|
| 121:51 | our F. Once we've done some and we know what the time |
|
| 121:56 | We measured the time in the lab that goes into our S equation. |
|
| 122:00 | figure out how much F. And from that we can back out |
|
| 122:03 | DNA squared was and take the log so that we get from the |
|
| 122:08 | this we get from the lab. know what temperature we heated today. |
|
| 122:13 | those are the laboratory values. And we then and and this again is |
|
| 122:18 | equation that tells us that stuff from from that solution of the differential |
|
| 122:24 | So that's from the experiment that that gives the F. Gives us the |
|
| 122:29 | . N. A squared and then tees from the experiment. And so |
|
| 122:32 | we can plot those values on a like that. And if everything goes |
|
| 122:38 | you get a straight line and then slope is gives you eat and oh |
|
| 122:44 | didn't I don't have it here. then the intercept wherever zero is, |
|
| 122:48 | give you did not on ice And this is why we call this |
|
| 122:52 | defensive Itty at infinite temperature because on access, whereas whereas infinite temperature when |
|
| 123:00 | when it's when one over T equals , one over T equals zero when |
|
| 123:07 | equals infinity. So that's the concept the diffuse it infinite temperature, obviously |
|
| 123:13 | exists an infinite temperature. But you extend that line to zero. And |
|
| 123:19 | now if we do this experiment, got everything, we need to calculate |
|
| 123:24 | the closure temperature is and now we understand aha. This system is the |
|
| 123:28 | of system that when we get an , we can say that that's when |
|
| 123:32 | rock was at this temperature. And are So everybody got that. That's |
|
| 123:39 | is where we were going, we're done. This is one, |
|
| 123:43 | can determine the value of a closure in the system and I think it's |
|
| 123:48 | it to go through this to give a sense that there is a thing |
|
| 123:51 | can be done. And then I'll show you some real data. This |
|
| 123:54 | something I did in my lab a years ago, I was interested in |
|
| 123:57 | helium diffusion and calcite. And so put some cow slide in the |
|
| 124:03 | we pumped it down and heated it in various temperatures heated from 100 degrees |
|
| 124:08 | 700 degrees. Here, it is reciprocal temperature. And the first few |
|
| 124:12 | here, make a really nice line square 2.998, you get a slope |
|
| 124:17 | that, calculate a closure temperature. , I should note that it is |
|
| 124:22 | standard case, I told you this that when you you have to, |
|
| 124:26 | you calculate the closure temperature, you to know what the cooling rate |
|
| 124:29 | Well, you never know what the rate is. You just have to |
|
| 124:32 | that the standard thing to assume unless have another reason Is that the cooling |
|
| 124:38 | is 10°C for a million years. just what we've all agreed on. |
|
| 124:42 | And so I'm reporting it here, know that the closer it'll be a |
|
| 124:47 | higher closure temperature. If it was , it'll be a lower closure temperature |
|
| 124:50 | slow, About 60°. That's a And so that's that now tells us |
|
| 124:56 | we want to use this system in geologic studies, it's only going to |
|
| 125:02 | sensitive when we get down to pretty temperatures, whereas other systems much |
|
| 125:09 | But that's what real data look like that we had to calculate the where |
|
| 125:14 | that? Um here's a different example a dolomite, same same study, |
|
| 125:19 | the same answer, 71°. Um And here something, we'll talk about tomorrow |
|
| 125:26 | a little bit tomorrow. It's a . These are results from a |
|
| 125:32 | This is argon Infeld. And you'll that the first few points here because |
|
| 125:39 | the first points are always on this , because this is low temperature side |
|
| 125:44 | championship, because it's a reciprocal. we always think of going up and |
|
| 125:49 | this way. So these low temperature make kind of a nice line. |
|
| 125:54 | Finley's fall off that line, well it pretty significantly. And why might |
|
| 126:01 | be? Well, it turns out feld Spars, a lot of work |
|
| 126:04 | gone into this Took a bunch of about 15 years to sort this |
|
| 126:09 | But what it is is that this to be an example of failed spars |
|
| 126:15 | . One of our rules is that not just one diffusion dimension. You've |
|
| 126:21 | you're a petroleum engineer. Have you at rocks in in microscopes? Look |
|
| 126:25 | that thin sections, Okay, well looked at farms and thin sections, |
|
| 126:34 | and thin sections have all sorts of things going on, twinning and and |
|
| 126:39 | sorts of different and and if you can look at them in different |
|
| 126:43 | different scales and feldspar are not at least optically. And it seems |
|
| 126:48 | the mathematics of the diffusion suggests that going on. Maybe it's the |
|
| 126:53 | maybe it's something else. But there's going on such that there are it's |
|
| 126:56 | just one dimension. And and the crystal, you know, the crystal |
|
| 127:01 | is not the diffusion, this is a, this is not the diffusion |
|
| 127:06 | , A is something smaller and in , there are big A's and little |
|
| 127:10 | inside of here. So there are sub growth sub domain sub grain domains |
|
| 127:16 | here which have a variety of sizes that's what's going on here. And |
|
| 127:20 | the early part we get the data get the gas coming out of the |
|
| 127:24 | ones are the easiest ones to remove from because they have the smallest distance |
|
| 127:28 | go. But the big domains hold their gas until later. And the |
|
| 127:33 | the Iranians diagram. This is this called the Iranians diagram because that equation |
|
| 127:39 | . Equals D. Not over That's called the Arrhenius equation. So |
|
| 127:44 | is this diagram is more complicated and probably because what's going on here is |
|
| 127:48 | got we've got some big domains, medium and some ones that are little |
|
| 127:53 | . And there's a whole bunch of and that goes into the modeling of |
|
| 127:56 | . But it has been shown for minerals, usually for argon and helium |
|
| 128:03 | that multiple diffusion might be a But that's a complication that we may |
|
| 128:08 | get too far into. Um But that's the mathematics and and of of |
|
| 128:17 | an age and the mathematics and the behind figuring out what that age |
|
| 128:22 | We have to do these laboratory experiments order to figure out what the activation |
|
| 128:28 | and the Dean on a square it . Once we have that, then |
|
| 128:31 | can go back to this first. . I didn't mean to do |
|
| 128:37 | Go back to this. Where did go here? This first slide. |
|
| 128:48 | . So this first slide. But one of these points here was coming |
|
| 128:53 | those sorts of experiments where you have do that business and figure out what |
|
| 128:57 | the diffuse city is at each step versus temperature. See if it's got |
|
| 129:02 | nice line from that. You can calculate the closure temperature and I remain |
|
| 129:07 | such that we see that the closure for many geologic systems go from as |
|
| 129:12 | as seven or 800 threes down to low as 50 or 60 degrees. |
|
| 129:17 | different geologic application. Right? Here's , these things, here are the |
|
| 129:23 | of things you might be in paying to if you're worried about whether |
|
| 129:26 | whether you're reservoir ever got hot enough make oil. These are the kind |
|
| 129:30 | things you might want to use because got some dyke cross cutting the |
|
| 129:34 | And you want to know the age the fall. If you don't want |
|
| 129:36 | deal with any of the possibilities of fact that these crystals might have been |
|
| 129:40 | up later on. Uh, so got a dike across fault. You |
|
| 129:46 | to know what the age of fault , right. The fault is older |
|
| 129:49 | the diet. You want to get oldest age you can on the |
|
| 129:52 | So you use this guy because it's gonna be heated by something metamorphic |
|
| 129:57 | But this one, if you were date the same diet as, as |
|
| 130:00 | appetite in it. And if you to date it by helium, you |
|
| 130:03 | do that. But it's not the choice if all you're interested in is |
|
| 130:07 | timing of the fault movement. If interested in when the fault moved and |
|
| 130:11 | how the whole thing evolved later on how it got to the surface and |
|
| 130:14 | blah blah well then do all of stuff. But if you were, |
|
| 130:20 | know, if you were in a , if you were a, you |
|
| 130:22 | a manager or you were asked a by a manager, you know, |
|
| 130:25 | are we going to figure out this ? Well what are we trying to |
|
| 130:29 | ? Figure out the age of this ? Are we trying to figure out |
|
| 130:31 | maximum temperature of the basin? Different . Different different approaches. Okay. |
|
| 130:40 | , so that's the end of slide number one And that takes us 3 |
|
| 130:46 | . Um I'm going to take another from my voice. Here is 3:18 |
|
| 130:53 | start again at Oh hell 3 Oh so what if I should resume |
|
| 131:14 | ? It's not doing anything. What I stop and start over? Is |
|
| 131:31 | okay? Okay. So I'm gonna gonna start sharing with our next, |
|
| 131:40 | next, So that's number two ice . Yes. Ah I can't remember |
|
| 134:07 | rangers to just finish with the rangers . It is the Angels. Okay |
|
| 134:15 | colors is pitching tonight. Um yes looking very good. Had the best |
|
| 134:21 | in the american league. So in , in the, for the playoffs |
|
| 134:25 | in a few weeks we'll get the one seed and and that means that |
|
| 134:31 | the Yankees will get the number two . But if and if the Yankees |
|
| 134:36 | in their series and the Astros prevail their series. The Astros will have |
|
| 134:40 | field advantage against the Yankees before they before the the uh All Star game |
|
| 134:53 | july july yes, they were neck neck. But since the, since |
|
| 134:59 | All Star game, the Yankees had stunk it up really bad. So |
|
| 135:05 | think at the out at the All game, the Yankees were three games |
|
| 135:09 | of the Astros. Now there's six behind is still out. But they |
|
| 135:20 | a new guy came up from triple lot this week pitched six innings of |
|
| 135:25 | hit of no runs. So that nice. But I think Verlander will |
|
| 135:31 | back, it's not a not his , it was his leg. Uh |
|
| 135:37 | , we've got the two games this , we'll probably go to one more |
|
| 135:42 | then I don't know, probably we've to the playoffs in the past but |
|
| 135:47 | hard to get tickets and they're very . But I did go that |
|
| 135:54 | The best thing I've ever been was year, it was the playoffs before |
|
| 136:00 | Dodgers against the Yankees and it was pitching on friday night and they had |
|
| 136:07 | had been in new york and lost couple of games. This was a |
|
| 136:10 | important game, they had to win Verlander pitched and it was the most |
|
| 136:16 | scene I've ever been in, you the place was packed of course the |
|
| 136:22 | was closed and there was just 40,000 who are all on the same |
|
| 136:26 | This was our tribe. 55 people don't know and everything. A good |
|
| 136:33 | . That was a, I used fifth game of the world Series as |
|
| 136:43 | example in some other things. I working in Arizona now for the last |
|
| 136:53 | years. Where in Arizona? I did my masters degree on the |
|
| 137:04 | in Pima County down by uh Well what's the name of that |
|
| 137:13 | Wilcox, East of Tucson. It's pretty ability to, we went to |
|
| 137:31 | games this year, but I think got covid at one of them. |
|
| 137:36 | . No, but because we're all everything. We went to the game |
|
| 137:42 | monday, thursday, I started feeling , tested on friday, got home |
|
| 137:49 | and it was positive. Uh, we managed to get the doctor to |
|
| 137:54 | us the prescription for those Covid drugs saturday afternoon. We got drugs and |
|
| 138:01 | was feeling pretty much better by the friday. So about eight days and |
|
| 138:08 | lot of people have said that I symptoms kind of like mine, they |
|
| 138:12 | a little bit, but mine was just a low grade headache and just |
|
| 138:16 | heavy fatigue for a week. you had fatigue. Yeah, my |
|
| 138:26 | my routine was, I'll try and this tv show and then I'm going |
|
| 138:29 | take a nap and then I'll have and then I'll take a minute And |
|
| 138:34 | about 7:30 I'm going to bed. I mean it didn't really, you |
|
| 138:40 | , I went I didn't have a , I didn't have a real |
|
| 138:43 | I just, I just know I'm to take a nap and then I'm |
|
| 138:48 | a small headache. When did when you do that? When were |
|
| 138:58 | when was your color? Oh before have lots of shots. Yeah. |
|
| 139:08 | . I talked to somebody yesterday. got covid before there were any |
|
| 139:12 | He was really sick. He takes hand. Are you from? I |
|
| 139:23 | up in Kansas. Okay. A of people just like us seem like |
|
| 139:36 | one of the most. Yeah, think that's going away a little |
|
| 139:45 | Well I think the whole sign stealing will go away. People will still |
|
| 139:50 | the actress because they win. Now can't blame the sign sign stealing |
|
| 139:55 | Are you a basketball man? I um, Kansas that was the |
|
| 140:03 | So those are my those are the two teams. I can probably tell |
|
| 140:07 | what you know what they did last what they're gonna do next is the |
|
| 140:11 | and the baskets. Yeah, Alrighty then. So we are sharing |
|
| 140:31 | . So we can just go to . That's correct. Right, you're |
|
| 140:34 | it. All right. So we're start talking about individual systems first we're |
|
| 140:42 | talk about something that has some sort good value for understanding things generally. |
|
| 140:50 | we won't use it a whole bunch we start talking about thermo chronology. |
|
| 140:54 | I think it's a valuable thing to about because we have to deal with |
|
| 140:58 | question of what if there were daughter to begin with? We've skipped over |
|
| 141:04 | problem for now. We're gonna come to it now because generally there are |
|
| 141:11 | are there are systems when there's always daughter president time equals zero. Let's |
|
| 141:15 | for example the rubidium strontium dating one of the earliest methods that was |
|
| 141:20 | in geo chronology. It's now not as much as it used to. |
|
| 141:24 | you may look at the literature that have, you know from the past |
|
| 141:27 | have these things and it's also similar other things that might be used more |
|
| 141:32 | , such as potassium argon MEREDITH, , Iridium, osmium which we'll look |
|
| 141:36 | Leticia happening, we won't talk But the concept of Cochran's is valuable |
|
| 141:41 | all of these things. So the of rubidium and strontium, rubidium is |
|
| 141:47 | lot like potassium strontium is a lot calcium. Um And because rubidium and |
|
| 141:54 | or potassium and calcium, they're You know they've got different charges. |
|
| 141:58 | got different radius but they're still not different. There's essentially no rubidium rubidium |
|
| 142:04 | minerals that are strong and free. that's the issue we gotta deal with |
|
| 142:11 | our from you know if we could say that did not equal zero, |
|
| 142:14 | finished de not is not equal to . So how are we going to |
|
| 142:18 | around that? Well, before we to that, let's just remember that |
|
| 142:22 | indicates to strontium it has a half of 48 billion years. You might |
|
| 142:27 | that's awfully long. How can we around? You know, is that |
|
| 142:32 | long that it's not useful? it's it's not although it is a |
|
| 142:36 | value, but this is the first , I guess I will drive home |
|
| 142:39 | point that we will try to remember and over again that from analytical |
|
| 142:45 | the only thing we have to worry is are there enough daughters to |
|
| 142:48 | Because there's almost always going to be parents to measure because we picked the |
|
| 142:53 | , right? We picked Zircon because has uranium in it. We picked |
|
| 142:57 | feldspar because it has potassium in we date courts because it doesn't have any |
|
| 143:04 | that stuff in it. So, know, if you try to date |
|
| 143:07 | , you're gonna have a problem. not enough parent, but if there's |
|
| 143:10 | enough parent, there's certainly not enough . Okay, so stay away from |
|
| 143:14 | , but if you pick the right , the parents not a problem question |
|
| 143:18 | is there going to be enough daughter measure? There's three ways you can |
|
| 143:24 | enough daughter to be in your One of them is if there's quite |
|
| 143:28 | lot of parent right, there's a of uranium in the zircon um what |
|
| 143:35 | there's not a lot of parents in , in your mineral, you can |
|
| 143:39 | , as long as there's some parents your mineral, what what what samples |
|
| 143:45 | be enough? Well, I'll if there's, if there's a little |
|
| 143:48 | of parent, not a lot, can still end up having a lot |
|
| 143:51 | daughter if the sample is very So just wait a while and there's |
|
| 144:00 | to measure. You can't measure, know, so if you have a |
|
| 144:03 | bitty amount of parents, you can't a sample that's, you know, |
|
| 144:07 | years old. But if you have little bit about a parent, you |
|
| 144:10 | measure something that's very real. And the third way to get around this |
|
| 144:15 | is just make the sample very you know, because we're not talking |
|
| 144:19 | total amount here. So we've got big sample now, big of |
|
| 144:23 | depends on the sensitivity of your machine even back 40, 50 years |
|
| 144:29 | the amount of rebellion and feldspar, know, there's quite a lot of |
|
| 144:33 | there. So you, you if you have a few milligrams of |
|
| 144:36 | , you'll be fine. Um so there's a lot of rubidium in feldspar |
|
| 144:41 | it's very easy to get, you , a bottle full of feldspar, |
|
| 144:45 | 48 billion years, not a We've, even if you noticed some |
|
| 144:51 | the half lives, I mentioned early that list of half lives, some |
|
| 144:55 | them are more than this. But anyway, here's our that's our |
|
| 145:00 | and there's two isotopes of rubidium um four isotopes of strong Notice that there's |
|
| 145:07 | one of billions radioactive assuming rubidium 87's rubidium 85 is not. And so |
|
| 145:14 | ratio of the two today is a number. That number used to be |
|
| 145:19 | in the past. Right? Because used to have more rubidium 87. |
|
| 145:24 | there's four isotopes of strontium, they're stable. But when we describe the |
|
| 145:31 | , we don't have quite the same decimal point precision because it depends on |
|
| 145:36 | material Because some material has lots of 27 and some doesn't it depends on |
|
| 145:43 | your material that you're looking at also a lot of rubidium in it and |
|
| 145:47 | or not it's very old. So you look at the salts that just |
|
| 145:52 | out of the mid ocean ridge, don't have very much 87 in them |
|
| 145:56 | all because they come from a specific rubidium tends to when the when the |
|
| 146:02 | melts more rubidium goes into the cross stays behind the mantle is rubidium pour |
|
| 146:08 | crust is rubidium rich. So if have a man, if you have |
|
| 146:12 | mantle melt today at the mid ocean , it's not gonna have much for |
|
| 146:16 | and of course it just happened So it's not gonna have much time |
|
| 146:19 | build up strong team anyway. Whereas you were to take a sample of |
|
| 146:23 | granite from from the R Key and , it's got a lot of |
|
| 146:27 | So these numbers are broad averages. numbers are not averages. These are |
|
| 146:34 | card numbers because you see 84, , 88 are are stable but they're |
|
| 146:40 | not growing. They're not they're not genic. Alright, so we got |
|
| 146:44 | ice tubs there. Three ice It's not moving. Start again. |
|
| 146:55 | it just stop a minute ago or it's okay? Uh It's moving |
|
| 147:06 | Okay, so where were we? were Here. Okay, so our |
|
| 147:14 | equation says that the amount of strontium we have today is equal the amount |
|
| 147:19 | strong in 87. We started with this equation which deals with the amount |
|
| 147:24 | Obsidian out of time. Right? get this. This is an equation |
|
| 147:28 | what I was just saying. How we get a lot of this? |
|
| 147:31 | don't have this number be big for number. Alright, that'll end up |
|
| 147:36 | over there of course. But we have this product. What the heck |
|
| 147:41 | that back when it all began? how we fix this problem. We |
|
| 147:46 | going to note that very unlikely for to be zero. We're just stuck |
|
| 147:51 | that. So we need a method determine this value. The method we're |
|
| 147:54 | use is we're going to say well got some other isotopes and it's the |
|
| 148:00 | that mass spectrometers, the machines we to do this stuff Are really good |
|
| 148:04 | measuring ratios. They're not they're not at measuring absolute abundances. So we're |
|
| 148:09 | we're gonna deal with ratios now and gonna toss in a normalizing factor of |
|
| 148:14 | of the isotopes of the daughter. just gonna divide the whole equation by |
|
| 148:19 | to 96. And now we've got equation that says the strontium 87-86 ratio |
|
| 148:26 | our sample is equal to that ratio it started plus this other business. |
|
| 148:33 | now you're thinking well you've just changed problem to a an abundance at the |
|
| 148:37 | to erase you at the beginning. has that helped? Here's how we |
|
| 148:42 | going to note that that value is we need to know but we can |
|
| 148:49 | advantage of the fact that in igneous there's a natural segregation of rubidium and |
|
| 148:56 | depending on what minerals you're talking Some minerals like rubidium like potassium |
|
| 149:01 | Remember rubidium goes with potassium strontium goes calcium. So our minerals like potassium |
|
| 149:07 | or bio type or muscovite is going have a lot of rubidium go into |
|
| 149:12 | . Whereas a mineral like plastic, or appetite. Those are calcium rich |
|
| 149:17 | . They'll have more strong. So ratio of those of those two elements |
|
| 149:23 | be different from a bunch of minerals all crystallized at the same time. |
|
| 149:30 | there is a process at least one action that's operating while these minerals are |
|
| 149:36 | there's no process that will fraction The isotopes of strong. So as |
|
| 149:41 | thing is crystal as these minerals are . Whatever strong team is going into |
|
| 149:46 | mineral will pay no heed to whether is strong from 87 or 86 they're |
|
| 149:51 | strong. And so whatever ratio you in the magma you will have that |
|
| 149:56 | ratio in the mineral in every So You will if you were to |
|
| 150:03 | that at this situation where we plot video 87 over strong 86 on this |
|
| 150:10 | 87 strong team over 86. Strong over there. Without to have this |
|
| 150:17 | the situation we would have at The minerals have just crystallized and they |
|
| 150:23 | a variety of rubidium strontium ratios. some minerals like some things and some |
|
| 150:28 | like others these are the potassium These are the calcium minerals. So |
|
| 150:33 | have a spread here but they have spread on the y axis because strontium |
|
| 150:39 | don't behave that way. All So this is where now we're on |
|
| 150:43 | way to fixing our problems. Um is at time zero as time |
|
| 150:51 | How will these points change on this ? Let's give you a little hint |
|
| 150:57 | is this is what's going on, 87 is going to decay to strong |
|
| 151:02 | center. How are these points gonna over time? What my water something |
|
| 151:12 | my water. Yes, that's my . Oh that's good. Let me |
|
| 151:27 | ask you how is rubidium 86 gonna over time 3-96 does not change. |
|
| 151:49 | only thing that change in the structure my Stopes is what what how how |
|
| 151:56 | it change over time? No, not talking. The abundance of strontium |
|
| 152:06 | is going to change over time in sample. It's gonna go up or |
|
| 152:12 | . It's gonna go up because rubidium became to produce estrogen and he said |
|
| 152:18 | 87 is radio genic 84 86 and are not. They're neither radio genic |
|
| 152:25 | radioactive. They're just there and that's it's good to use them as a |
|
| 152:30 | thing because they run variant. So go back to this thing with rubidium |
|
| 152:42 | rebellion saying to scratch him. How these things gonna evolve? What how |
|
| 152:47 | this race? You're going to change time? Thank you. No, |
|
| 153:00 | , I mean rubidium is radioactive. still always going to be a radioactive |
|
| 153:06 | but over time we're gonna lose some it. Right? Just like we |
|
| 153:12 | coins and eventually everyone in the stadium to sit down. Eventually we'll run |
|
| 153:17 | of rubidium 87. It will all strontium 87. And while that's |
|
| 153:21 | what happened here to the strontium Nothing. So what happens in this |
|
| 153:37 | ? Yes, this is going this is staying the same. It |
|
| 153:42 | down, What is that gonna Mhm And the strategy 96 will be |
|
| 154:10 | . So that quote will go that's going up, that's going in |
|
| 154:16 | will produce a trajectory on this That looks like that. That makes |
|
| 154:27 | . The table of isotopes. Four those three of those isotopes are just |
|
| 154:41 | . They are the same that we got all the strong in 88. |
|
| 154:44 | ever gonna have it's not getting It's not getting smaller. Just like |
|
| 154:49 | of the isotopes on the on the table. They're you know, they're |
|
| 154:53 | radioactive or radio genic. Just a . You know These lectures I'm giving |
|
| 154:57 | you are about some rare cases is radioactive element. It makes strong community |
|
| 155:05 | go forward now. Okay. And this happens. Why are those arrows |
|
| 155:15 | those lines with the arrows on Not the same length singing. Those |
|
| 155:32 | different minerals, appetite, plastic, , bio tight K feldspar muscovite. |
|
| 155:37 | different minerals. Well, that's what , but that's why they're different. |
|
| 155:44 | why they fought on a line. but why is this line longer? |
|
| 155:49 | as long as this one. This like seems to have changed a bunch |
|
| 155:54 | these guys didn't change very much think . They're both just as unstable. |
|
| 156:02 | is we all have the same quarters our pockets. But this it's the |
|
| 156:11 | game and this is our classroom, why is there more change here? |
|
| 156:20 | there's more of it here. I get it so that we would predict |
|
| 156:28 | this life has to be barbara because number of decays is proportional to the |
|
| 156:34 | of parents you have. And if out on this side of the |
|
| 156:37 | we've got more parents, you're down , you shouldn't expect it to move |
|
| 156:41 | much because you ain't got much. so we would expect these things to |
|
| 156:46 | to some line like this. And it's a nice straight line, we |
|
| 156:49 | talk that right in there and the . one then is the age of |
|
| 156:52 | system. And we can calculate the based on the slope of that |
|
| 156:59 | The system starts out with a horizontal and over time rotates to become steeper |
|
| 157:04 | age. And now we've got this of the system and that's how we |
|
| 157:08 | around the problem of not knowing what initial value is. Now. In |
|
| 157:11 | we get the initial value, that's slow, that's the initial value. |
|
| 157:16 | we can expect nor the initial value we're not caring about it very much |
|
| 157:20 | the age comes from just this but we had to have a spread |
|
| 157:24 | these values in order to make this but we got, so this is |
|
| 157:30 | ice crown approach ice cream means equal that's the ice across online. And |
|
| 157:37 | we start out with the assumption that will be a spread in parent to |
|
| 157:41 | ratio, but not a spread in isotopes of the daughter. This all |
|
| 157:48 | from that and this is the way you will in general avoid or solve |
|
| 157:55 | problem of, oh my gosh, many daughters were there at the |
|
| 158:01 | Don't know how many, but the is sorted out with this. And |
|
| 158:05 | I said, sometimes the ratio we use for valuable information. Sometimes we |
|
| 158:11 | . But the age has come straight that. Let me just show you |
|
| 158:14 | real data. Here's a here's an from central texas. The llano |
|
| 158:22 | Their, you know, just west Austin has a bunch of old rocks |
|
| 158:25 | it. This is from a we've got a K feldspar, whole |
|
| 158:29 | muscovite and a bite attack. Notice the range of this value goes up |
|
| 158:33 | about 900 huge spread between these That's good. The more spread we |
|
| 158:38 | more confidence we have in the If these points all over on top |
|
| 158:41 | each other, they wouldn't make much a line. So nice to have |
|
| 158:45 | bio tied and the case bar and same thing, big dip. That's |
|
| 158:49 | and this is the same age we'll for a bunch of different rocks out |
|
| 158:53 | . It's been shown that the Llano is about 1080. Okay. And |
|
| 159:01 | morning we'll look at I think a really near here with a different ice |
|
| 159:06 | system gives us today. Um Here's example. This comes from a lunar |
|
| 159:16 | it. Remember what it done It Tonight is an igneous rock that's almost |
|
| 159:22 | all of the and olivine is a mineral. There's not you know this |
|
| 159:31 | a lot of rubidium in in in assault or of all of the you |
|
| 159:36 | these are different whole rocks or pieces ah levin in this but notice that |
|
| 159:41 | X. Axis on the other one to 900. The x axis only |
|
| 159:46 | 2.2 data are pretty packed together But we still get to resume in |
|
| 159:52 | look at the state and we still a pretty good line here. Why |
|
| 159:56 | this rocks 4.5 billion years old. is what I told you that even |
|
| 160:00 | this value is quite low, it's got a pretty good line here because |
|
| 160:05 | waited a long long long time. you can date anything from the moon |
|
| 160:10 | they're all very old. You would want to take a rock that was |
|
| 160:15 | know 100 million years old. That would never want to date a done |
|
| 160:19 | that was 100 million years old. this technique because that all the points |
|
| 160:22 | basically be on top of each other be really hard to tell. But |
|
| 160:25 | with four billion years we see a , it's okay, but same |
|
| 160:32 | Um So that's what, so with icy Cron, you know, we |
|
| 160:38 | to assume that there hasn't been that whole thing has been a closed system |
|
| 160:42 | we haven't had a lot of, know, alteration or heating, but |
|
| 160:45 | than that it works out pretty Um Actually I think I'll skip |
|
| 160:51 | I'll just point out one thing that can also, we do have to |
|
| 160:55 | concerned if you were wanting to date say where you were having some look |
|
| 160:59 | some strata graphic sequence and you got basement rock and you need to know |
|
| 161:03 | old that basement is to look at base in history or something like |
|
| 161:07 | You might think, well let's do for and that's not so bad. |
|
| 161:11 | . Um but you but you need know that this this system can be |
|
| 161:19 | by metamorphic effects. If the heating high enough or long enough, the |
|
| 161:24 | isotopes will homogenize throughout the rock. that here we've got our here's an |
|
| 161:31 | that we just described in our last example, if things get hot. |
|
| 161:36 | if we metamorphose this system, this happen that all of the strong team |
|
| 161:42 | start talking to each other and then gets all nice and quick liberated and |
|
| 161:46 | we get that and then that's basically time of metamorphoses um now these things |
|
| 161:52 | going to evolve away and and so see the the original igneous system of |
|
| 161:59 | out because we don't get to know that because that metamorphoses um resets it |
|
| 162:06 | . They start heading off in that . We get some tea to that |
|
| 162:10 | the time since metamorphoses um that how , how much, how much time |
|
| 162:16 | before that in What's what's what's one and T0 that's gone now. |
|
| 162:23 | , now if all you're interested in the age of basement for how, |
|
| 162:26 | old were these rocks when it this probably better because the metamorphoses um is |
|
| 162:31 | to be younger than the age. just so you know, for metamorphic |
|
| 162:35 | we're looking at the time of metamorphose if it was hot in them. |
|
| 162:39 | so here's an example, also look the strontium isotope evolution. If we |
|
| 162:44 | at the 87, rations or versus , you know, imagine we had |
|
| 162:48 | sort of granite with the appetite and rock and case bar and bio |
|
| 162:52 | They would start out at some common and move up, goes up really |
|
| 162:57 | and have to tighten up very much then metamorphose um brings them all together |
|
| 163:02 | then they head off again like Um Here's an example of a real |
|
| 163:09 | , This is a nice from labrador again we don't see very much spread |
|
| 163:15 | , but it's enough because this rock 2.7 billion years old. And we |
|
| 163:19 | interpret this not as the age of pro to lift, but the age |
|
| 163:24 | the time of metamorphosis. We'll see later on. If we want to |
|
| 163:29 | who want to see through this um We're gonna have to use systems |
|
| 163:33 | won't be reset by this metamorphosis. will be that will be uranium |
|
| 163:38 | that will be uranium lead in We can date this rock by uranium |
|
| 163:43 | , we'll get 3.5 billion something like because the metamorphoses um you know, |
|
| 163:49 | Zircon just laughs at this metamorphoses. Whereas the rebellion scrunch him, it's |
|
| 163:54 | re equivalent. And that concept will the same. Whether you're talking about |
|
| 164:00 | temperature metamorphic ism or medium temperature basin , you've got to pick the right |
|
| 164:05 | to know whether you've been reheated, or sometimes you want to know when |
|
| 164:09 | reheating is. If you're a metamorphic , this is what your answer |
|
| 164:13 | If you're interested in the petroleum maturation this system, you don't really care |
|
| 164:18 | old the rocks are, you care . Did they get hot enough? |
|
| 164:20 | gotta pick the right system to tell that um this is a kind of |
|
| 164:26 | example. It's a really old but it shows that the the ICC |
|
| 164:30 | abrasion of systems like this depend on here, we have a bunch of |
|
| 164:36 | from Scotland and in red are plotted strontium data of whole rocks that take |
|
| 164:44 | rock and grind it up and homogenize . That's just a powder of the |
|
| 164:49 | . And they plop that on this a crime they get in the age |
|
| 164:52 | 4548. And these are the points . But if you look at the |
|
| 164:58 | from just this rock here, you it out and look at minerals, |
|
| 165:02 | minerals of the rock. The boat , here's the tragically here's the whole |
|
| 165:07 | must fight. That gives them much slope and an age of 400 |
|
| 165:14 | Whereas these give an age of So what's going on here? |
|
| 165:20 | it looks like on this. And these these coal rocks were probably collected |
|
| 165:24 | outcrops, you know, that are of meters apart, maybe more than |
|
| 165:30 | . But the minerals were all collected Iraq. And so what this says |
|
| 165:35 | on the scale of the hand the metamorphoses um re equip vibrates everything |
|
| 165:40 | puts a new slow part. But the scale of these hand, specimens |
|
| 165:45 | were collected, you know, hundreds meters apart, they did not re |
|
| 165:50 | break. And we looked at just isotopes in this little system versus this |
|
| 165:53 | for that. So we can say these initial age of this system was |
|
| 165:58 | 50 then it was re equip rated 400 and we can do that with |
|
| 166:04 | same video instruction system, but only we look at parts that have different |
|
| 166:09 | variability. That's correct. Um What the temperature that this happens at? |
|
| 166:18 | don't know really well, but it's around 300°. So we can we can |
|
| 166:23 | that re equip vibration and that that beginning of a new system, 3 |
|
| 166:29 | fish or something like that. Um we want this isn't too important for |
|
| 166:39 | guys, but it's just go through quickly. The the the the the |
|
| 166:47 | can be valuable. We can talk it yet. Just worry about the |
|
| 166:50 | here. But this intercept can be to tell you about the kind of |
|
| 166:55 | we're dealing with. And if you're in broad understanding of continental growth or |
|
| 167:01 | ice a topic or the plumbing of igneous system might come in handy. |
|
| 167:06 | is a data from a from a Celtic contracts kind of meteorites and you'll |
|
| 167:12 | that this back, these are the primitive rocks that we know about. |
|
| 167:19 | this has an initial value here, . And this is what's referred to |
|
| 167:27 | baby, which stands for basaltic, con dry best initial. They've never |
|
| 167:32 | a strong from 87 86 initial value than this. That's just as low |
|
| 167:37 | it gets. But if you if have a brock with lots of rubidium |
|
| 167:42 | you let it sit around. I this is an old rock, it's |
|
| 167:45 | billion years old, but it still this low value. But other |
|
| 167:49 | you know, take a look at rock here. This is a granite |
|
| 167:52 | diary from uh from china. And has an intercept .711 find a lot |
|
| 168:01 | . What does that tell us? , that tells us that the rocks |
|
| 168:03 | melted to produce this granite which is million or so, the rocks that |
|
| 168:08 | to produce that had evolved by a . They were not. This rock |
|
| 168:12 | come straight from the mantle. This reheated continent because the only way that |
|
| 168:16 | could have a strong team isotopes that who have already concentrated the rubidium for |
|
| 168:21 | long time. Um And here's an of a rock from Argentina. That's |
|
| 168:28 | same age for 70, but has much lower intercept. This is |
|
| 168:35 | This suggests a tectonic environment in which is coming pretty much straight from the |
|
| 168:41 | . So in sorting out your tectonic of your overall regional geology, this |
|
| 168:47 | you that, you know, the is pretty deep going down to the |
|
| 168:51 | , whereas that other granite was just melting crust. So there's some tectonic |
|
| 168:56 | at that. Um And then one thing about that we can use is |
|
| 169:03 | , you know, again, because is more incompatible than strontium. The |
|
| 169:08 | strontium of the melt is greater than source. And so when that crustal |
|
| 169:12 | ation takes place, we got more in the crust than the mantle. |
|
| 169:17 | then if we let that crust sit for a long time, that rubidium |
|
| 169:21 | decades too strong to 97. So can expect that ratio to be higher |
|
| 169:24 | the crust than in the mint. generally we place that sort of boundary |
|
| 169:29 | 706 In these rocks that have an value of 706 came from the |
|
| 169:36 | It was less than that. There a strong mental component and we can |
|
| 169:40 | this in North America. It's been that for igneous rocks here in Western |
|
| 169:44 | America, this green line, it's of hard to see. That's the |
|
| 169:49 | line Lines were igneous rocks to the of that. The initial value ratio |
|
| 169:55 | greater than 706 To the west of , about the initial value is less |
|
| 170:01 | 706, suggesting that rockets. This the this is the edge of the |
|
| 170:06 | old. All of these rocks out have been added to the product of |
|
| 170:11 | activity in the the area that has called suspect terrain. All been added |
|
| 170:17 | and that's consistent with the geochemistry of rocks. These rocks are much more |
|
| 170:22 | closely related to mantle derived activity. we can see a whole sort of |
|
| 170:26 | in the continent by understanding the initial . Um and this is from rocks |
|
| 170:32 | many different agents here, we're just at the intercepts. Um, one |
|
| 170:38 | thing about isotopes and this gets back more sedimentary advantages is that again? |
|
| 170:44 | high 87 86 reflects old frenetic source . And if we're eroding that frenetic |
|
| 170:52 | area rapidly, it's going to affect chemistry of the oceans because it's been |
|
| 170:56 | that the 87 86 ratio of modern is invariants, which is to say |
|
| 171:04 | team is well mixed in the throw some strong in the ocean. |
|
| 171:07 | mixes up pretty quickly and the organisms live in the ocean take on that |
|
| 171:12 | . A topic value from the water live in. And so we can |
|
| 171:15 | how the strontium isotopic ratio of the have varied over time by looking at |
|
| 171:21 | fossils and We can then hope to truncheon isotopes of fossils to tell us |
|
| 171:30 | about strategic. Perhaps it would be if that 87-86 ratio was changing rapidly |
|
| 171:35 | it hasn't been modified. But here's some people have shown here is a |
|
| 171:42 | of the 87 86 ratio in marine over time. And you see it |
|
| 171:48 | up and down and up and down it's not very good for strata. |
|
| 171:54 | determination until we get to the young . But before we talk about |
|
| 171:58 | we can just say that. What this tell us about? What does |
|
| 172:01 | up and down tell us? Why the strontium 87 86 ratio of the |
|
| 172:06 | tend to go down or tend to up Remember? Where is, where |
|
| 172:12 | , where is there are lots of strong team and where is there |
|
| 172:25 | Go back to this slide here. got rubidium is more incompatible than |
|
| 172:32 | The rubidium strontium of a melt is than that of its source. So |
|
| 172:37 | we melt the mantle and push some that stuff into the crust, we're |
|
| 172:41 | up more rubidium strontium. We let sit around in the crust for a |
|
| 172:46 | . That makes a lot of strontium Strontium 87 is a crustal source crustal |
|
| 172:54 | . And so what would then make value go up or down? This |
|
| 172:59 | remember a global average. These are are fossils that lived in the ocean |
|
| 173:04 | the ocean is well mixed. So would cause the global average of strontium |
|
| 173:10 | to go up or down in the ? Well, what kind of volcanic |
|
| 173:26 | , huh, tsunami? No, just moving water around. That's not |
|
| 173:37 | the composition of it. Okay, does that happen? Where do you |
|
| 173:52 | mantle and oceans interacting really good at mid ocean ridges. Got new mantel |
|
| 174:04 | up and melting and making ocean crust there. That's an addition from the |
|
| 174:09 | . So if you have really rapid floor spreading. Like when we're making |
|
| 174:15 | atlantic ocean spreading really quickly? How that show up on this graph? |
|
| 174:26 | would go down because you have a have more contribution of rocks that have |
|
| 174:31 | values. But what happened? So that's the way in which you could |
|
| 174:35 | it down. We're interacting the we're having the inter ocean interact with |
|
| 174:39 | bunch of low strong 87 rocks. can we make this graph go |
|
| 174:45 | And it's not just by slowing that down. So we can we can |
|
| 174:55 | the chemistry of the ocean from from bottom of the ocean. We can |
|
| 174:58 | , you know, we change the of the ocean by change the chemistry |
|
| 175:03 | your tea by putting sugar in How are we gonna change? That's |
|
| 175:09 | clue for changing the ocean. they don't have people. I |
|
| 175:20 | it couldn't have to be about, not necessarily volcanic rocks. What's what's |
|
| 175:24 | more common way of putting stuff into ocean than volcanoes happening right now? |
|
| 175:32 | , even better. I mean the doesn't change the chemistry of the |
|
| 175:36 | There's not much chemistry here. Oh no. I mean how do |
|
| 175:42 | get stuff into the ocean? Rivers so where do the rivers come |
|
| 175:56 | Okay, mountains, but mountains are mean it could even be not |
|
| 176:01 | but what's the continents, continents are you had a big if you had |
|
| 176:09 | . And so you need erosion of continents to bring this old Dravidian rich |
|
| 176:14 | back into the oceans, How would get? How would you increase |
|
| 176:28 | Two waves. Excuse me. windy I mean but I mean that's |
|
| 176:39 | way erosion happens. Wind is one is another. Um But how would |
|
| 176:44 | change the rate? Why would why the the rate of erosion in the |
|
| 176:48 | the world go up or down? because we're saying that basically if this |
|
| 176:53 | going up like this, this might the fact that more continental erosion |
|
| 176:58 | Because because this value is going this is the addition of oceanic castle |
|
| 177:03 | that value down. Continental erosion draws back, pushes it back up. |
|
| 177:09 | makes, what makes continental erosion Is erosion faster in southern India or |
|
| 177:19 | India? Way up there in northern . What do you got there? |
|
| 177:30 | . Is that a place of What about in in southern? What |
|
| 177:35 | in salon? Is that is that place of erosion? Yeah, because |
|
| 177:39 | flatter down there. So you can more erosion by making mountains. So |
|
| 177:45 | would indicate may be a time of mountain building. This is a time |
|
| 177:49 | acting ocean spreading. Of course that happen at the same time. And |
|
| 177:53 | can even each other out. But then just skip ahead to this |
|
| 178:00 | Now we have we just look at last 70 or 80 million years. |
|
| 178:04 | can actually see that we could actually we didn't if we had some |
|
| 178:08 | let's say but they weren't very diagnostic we could Figure out there 87 86 |
|
| 178:16 | say aha we know that these rocks post cretaceous let's say but we're not |
|
| 178:21 | exactly where the fossils are fossils. all the fossils tell us. Is |
|
| 178:26 | post cretaceous? Well they were That would mean 28 million years |
|
| 178:34 | So you can use the isotopes in section of the curve. Tell you |
|
| 178:38 | even you know, it's nice, steep line here. You can 7085 |
|
| 178:43 | 7084. That's a couple of million difference. You can use this as |
|
| 178:47 | strata graphic tool that you had nothing . If you had no inter bedded |
|
| 178:52 | rocks. If you didn't have the very well. If you were pretty |
|
| 178:56 | that this was less than cretaceous, would be a way of figuring that |
|
| 179:01 | Now. Why? What is the is the reason for 650 million years |
|
| 179:06 | that curve is so steep and so . We already discussed the answer. |
|
| 179:16 | mountains? No, I don't mean kind of mountains, I mean which |
|
| 179:23 | on earth? All the mountains are continents. It was a mountain |
|
| 179:36 | We discussed a few seconds ago. . The Himalayas are the biggest |
|
| 179:41 | They're delivering the most sediment to the . So they are and they're full |
|
| 179:45 | old rocks. The Himalayas have our and rocks in them some younger rocks |
|
| 179:51 | they have a lot of old rocks know all of the indian shield is |
|
| 179:53 | right? And now it's being pushed in parts of the Himalayas and eroded |
|
| 179:58 | the down the Ganges and the Indus . That's delivering lots of set into |
|
| 180:02 | ocean, changing the chemistry of the because we are taking a big high |
|
| 180:07 | roading it like crazy and pushing Material the ocean, changing the chemistry. |
|
| 180:13 | that's why since about 50 million years is consistent with all of our knowledge |
|
| 180:17 | the tectonics of the Himalayas. This gone up like crazy And that makes |
|
| 180:23 | for a good strata graphic tool. you've got nothing else and you know |
|
| 180:27 | rocks are less than 60 million years . What do you need to |
|
| 180:36 | Rubidium strontium dating? Almost any sample be fine. And nowadays these modern |
|
| 180:41 | spectrometers can can do something very well down to just really good with much |
|
| 180:47 | than a milligram of a sample. spread of rebellion strong and will be |
|
| 180:52 | for a good ice clock. You just get a bunch of points on |
|
| 180:54 | of each other because they will just to be still on top of each |
|
| 180:58 | . You need to spread so good nice line and it's difficult to take |
|
| 181:02 | less than 20 million. I mean people sometimes you've done better than |
|
| 181:08 | You know and and this this I this slide a long time ago. |
|
| 181:12 | should I should update it, you , maybe five million these days. |
|
| 181:15 | because the half life is 48 billion old, there is some limit to |
|
| 181:20 | , but 15 million probably just So, I mean the good news |
|
| 181:25 | that for something like this, you the way the machines have moved |
|
| 181:28 | This is not a problem. You a sample, a small piece of |
|
| 181:32 | , bit of feldspar, it'll be . I think that's the end of |
|
| 181:39 | . No, remedial mosby, I we have a second Cron system that |
|
| 181:44 | some applications to sedimentary rocks. I'm to talk about, I'm gonna talk |
|
| 181:48 | samarra, neodymium or lubrication, half um those are mostly igneous problems. |
|
| 181:53 | this Rania mosby um has been used for or minerals or meteorites, but |
|
| 181:59 | it's been used for shales. Medium 1 87. The case in |
|
| 182:05 | in 1 87 by beta decay. Vini um molybdenum and osmium are all |
|
| 182:13 | of platinum group elements. There, broadly cetera file, they go with |
|
| 182:19 | , that's why they're finding in iron and things like that. Uh the |
|
| 182:25 | life is again a long 1 43 , but you get a few places |
|
| 182:31 | they are concentrated, that's not a . Uh there's two isotopes. So |
|
| 182:36 | says rubidium up there should say excuse me. Um and there's a |
|
| 182:42 | of isotopes of osmium. We're gonna the same thing again, we've got |
|
| 182:45 | equation osmium um amount of oz being have depends on how much we started |
|
| 182:51 | . And so we'll just do the sort of thing again, will normalize |
|
| 182:56 | same story. Okay, we just different, just different values here, |
|
| 183:01 | it's the same idea. But Why? It's interesting is that it |
|
| 183:07 | been shown for organic shales that the osmium ratio in these shales is very |
|
| 183:16 | dependent on what kind of organic Species have. And even in a |
|
| 183:22 | even within a single basin or even a few outcrops, there's enough |
|
| 183:27 | You see this variation goes from 30 huge value. That's enough. There's |
|
| 183:32 | about the organic material that will preferentially Renea Moresby and that's important, |
|
| 183:39 | Because I said if you have, if if Renea mas ni um fell |
|
| 183:44 | the shales at the bottom equally. all plot on the same place. |
|
| 183:48 | wouldn't be valuable, but for some they are fractionated in some way that |
|
| 183:52 | to have something to do with the of organic activity or the kinds of |
|
| 183:58 | , organic molecules. I don't know about it. But what this, |
|
| 184:02 | this study showed was that you can pretty good results. Here's, here's |
|
| 184:07 | , here's some shales that were deposited the Appian album boundary, which we |
|
| 184:12 | know is about 100 and 12 million ago. So you look at those |
|
| 184:16 | , you plot them on an icy diagram, you get 100 and nine |
|
| 184:21 | or minus six now, medium not as you know, there's a |
|
| 184:26 | of issues with measurement and and so said that plus or minus five million |
|
| 184:32 | add, well, this is an . This, this they're doing as |
|
| 184:36 | as they can right now. If if this was, if you were |
|
| 184:38 | a real light by Iranian led, would be rejected answer no. Plus |
|
| 184:43 | minus six, go back and do good job, but plus or minus |
|
| 184:47 | million years old for medium not so . This was more of a proof |
|
| 184:50 | concept to say, look Suppose we know the age of the shale. |
|
| 184:55 | know, once again, we're trying develop systems for when we, you |
|
| 184:59 | , we don't have inter bedded volcanic , we don't have good fossils. |
|
| 185:04 | , strontium isotopes are no good because older than 60 million here. |
|
| 185:10 | let's try the Media mas mia. we got basically the right answer. |
|
| 185:14 | know, within a few million Here's the same study where they looked |
|
| 185:17 | a little bit younger. The timony, cinnamon Ian, Tyrone |
|
| 185:21 | that's 94 million. They got 92 . So it is a way to |
|
| 185:27 | shales directly direct dating of sedimentary rocks pretty much unheard of, right? |
|
| 185:33 | because because because because chemical sedimentary rocks lack sufficient concentrations of the parents. |
|
| 185:44 | no uranium, there's very little there's very little potassium in lime |
|
| 185:50 | So they're not good candidates for dating . Plastic rocks are full of material |
|
| 185:57 | older than the rock they came from else. Right? So dating, |
|
| 186:00 | plastic material just tells you how old provenance was. But here this medium |
|
| 186:07 | Bosnian come out of the water in proportions based on the organic activity in |
|
| 186:13 | water and then it produces the spread we can date this shale direct. |
|
| 186:19 | rare. So it's worth talking about Here's another example from the protozoa where |
|
| 186:24 | don't have fossils and I got an of, what was it? 7 |
|
| 186:30 | plus minus six on the radium, of this shale. And that's probably |
|
| 186:35 | better than you're ever going to get protozoa fossils if you find a fossil |
|
| 186:40 | all and a rock like this. that's that's the end of that. |
|
| 186:48 | , 4 15, we can probably a little more time on stuff. |
|
| 186:54 | So I'm going to stop that share I will start another oh, I |
|
| 187:03 | let's go to my power point, rid of that and we'll go to |
|
| 187:12 | power point and we'll get rid of . Open up our 3rd slides |
|
| 187:21 | we're going to talk next about uranium dating. So from now on, |
|
| 187:36 | gonna be talking about various systems for minerals. Um And we're gonna we're |
|
| 187:43 | go in order from highest closure temperature lowest closure temperature. We're gonna start |
|
| 187:48 | uranium lead. We'll finish off with helium Now uranium decays to both lead |
|
| 187:55 | healing same thing. But by but we get, when we get the |
|
| 187:59 | of uranium to lead, we get answer. When we do it from |
|
| 188:03 | two helium, get another answer. of the different closure temperature. Helium |
|
| 188:08 | small, lead is big, hard move around. So you've got to |
|
| 188:12 | to a high temperature to make I mean, I should say it |
|
| 188:17 | a closed system at a relatively high , whereas helium will not become a |
|
| 188:22 | , closed system until you get to a low temperature. So we'll get |
|
| 188:26 | helium last because it has the lowest temperature. We're gonna take these in |
|
| 188:30 | of closure. Starting with uranium Uh It's one of the best ways |
|
| 188:39 | determine crystallization ages of igneous minerals and it. And that's because of its |
|
| 188:45 | high closure temperature. If you want know when a mineral crystallized, this |
|
| 188:49 | great because none of the other things can happen to a mineral are going |
|
| 188:52 | be affected here metamorphoses. Um And it's zircon, remember zircon is very |
|
| 188:58 | to weathering. It's just as durable quartz in a sedimentary environment. You |
|
| 189:02 | melt it in an igneous environment. say diamonds are forever, but courts |
|
| 189:06 | more so, I mean diamonds are . Zircon even more. So so |
|
| 189:12 | why. And it's it's got this got a really high closure temperature and |
|
| 189:16 | really stable. Um That's Zircon, are other minerals we might use that |
|
| 189:21 | also used here but they're all accessory in rocks like granite that have high |
|
| 189:27 | concentration minerals like Mona's I appetite through any teams being all are relatively high |
|
| 189:34 | uranium. Let's look at the chemistry these systems. Oh and we're also |
|
| 189:40 | talk about korean thorium is another thorium decays to lead. So it's |
|
| 189:46 | in the same family and it has and uranium are chemically very similar. |
|
| 189:51 | we'll talk about thorium a little bit . Um Although generally the geochemistry of |
|
| 189:58 | thorium is more difficult so people don't do it, uranium will be |
|
| 190:02 | But there's a reason why we have pay attention thorium for a special case |
|
| 190:06 | we'll talk about later. Um so I've listed here uranium thorium lead |
|
| 190:15 | Zircon, the reason I mentioned Zircon because we're gonna not not zircon zirconium |
|
| 190:22 | we're gonna talk about zircon a And so looking at the ionic characteristics |
|
| 190:29 | these of these elements. Zircon has ionic charge, Zirconium has an ionic |
|
| 190:35 | of plus four tends to give away electrons. And when it does so |
|
| 190:39 | makes a I it makes an eye that has a radius of about |
|
| 190:42 | Angstrom. Look at uranium, It's similar, it also has a charge |
|
| 190:49 | Plus four and an ionic radius just little bit bigger. Now, you |
|
| 190:54 | from mineralogy when we're making an ionic , what's the most important thing in |
|
| 191:02 | these things? Is it the charge the size in deciding whether it's a |
|
| 191:07 | fit. When we make a for example, charge you said |
|
| 191:15 | I couldn't hear you, I'm Okay, well you were right speak |
|
| 191:23 | . Charge is more important because what is the rule when we were |
|
| 191:26 | out of mineral Z. R. . I. 04, that has |
|
| 191:29 | do so that those that has to something that has to add up to |
|
| 191:34 | take the charges of Z. S. I. 04 or any |
|
| 191:37 | . The charges of those individual atoms to add up to what I have |
|
| 191:43 | add up to zero. Remember you've you've got an ions, you got |
|
| 191:46 | ions. The whole thing has to up to zero. That's a rule |
|
| 191:50 | there's no there's no shimmying out of . zero is the only option. |
|
| 191:56 | size thing. A little more If you if you stuff something into |
|
| 192:01 | hole that's a little too big or little too small, you have a |
|
| 192:04 | have a perturbation of the lattice. might, you know, you'll have |
|
| 192:07 | some optical properties may change, but still a mineral. So the more |
|
| 192:12 | is the charge uranium has the same the opponent and the and the size |
|
| 192:18 | . So you want. So when making a zircon out of a |
|
| 192:22 | we're building it. We got we've got silica, we've got putting |
|
| 192:25 | together. Oh here comes the That'll do just fine. Put that |
|
| 192:29 | the zirconium spot. Same thing with . You can put that in there |
|
| 192:35 | . But look at that, that a charge of plus two. That's |
|
| 192:39 | off of our rules. We can't a plus to an A plus four |
|
| 192:43 | and it's a lot bigger too. we should not expect to get any |
|
| 192:52 | in a zircon at the beginning. is this is the example of where |
|
| 192:57 | know we don't worry very much about amount of glad that's in a zircon |
|
| 193:02 | the beginning. We can just say of these characteristics, let's just call |
|
| 193:06 | zero. So when we find lead the zircon it's because the uranium decayed |
|
| 193:12 | put it there. Okay, that's that's that's why that's another reason why |
|
| 193:18 | like so much is we don't have mess with this initial condition business. |
|
| 193:24 | , so there's a bunch of isotopes uranium. They're all radioactive some very |
|
| 193:30 | . We're not going to pay attention those other ones very much. Um |
|
| 193:35 | two are both the other two are but one has a half life of |
|
| 193:39 | billion. One has a half 704 million. So that means today |
|
| 193:43 | ratio of those two is 137.88. course. That ratio is different in |
|
| 193:48 | past. That's today's ratio. Um got to race at two. We've |
|
| 193:54 | a nice thing going on here is we've got two isotopes of one thing |
|
| 193:58 | to two isotopes of. Another we can really pay attention to them |
|
| 194:02 | ways that we can't from these other . So first, let's look at |
|
| 194:08 | 2 38 case to electro six plus helium And it has a half life |
|
| 194:15 | 4.4, 7 billion years. But energy you're 18 to 35. The |
|
| 194:21 | led to a seven plus some helium some energy half life 704 million. |
|
| 194:28 | One thing I always had trouble with I was a student, like, |
|
| 194:32 | know, there's two uranium and two , which one goes to, which |
|
| 194:35 | remember the even one goes to the one and the odd one goes to |
|
| 194:38 | odd 206 - 8 - 30 acres - $35 - 207. And that's |
|
| 194:46 | well that just is because um and we don't is even more simple because |
|
| 194:53 | just one isotope of thorium that's got sort of long half life and it |
|
| 194:58 | a simple decay at the coast decays lead 208 plus six helium. It |
|
| 195:03 | a half life of 14 billion so it's longer, but still you |
|
| 195:07 | use that. Um There are a of isotopes led These three in italics |
|
| 195:15 | the ones that are radia genic. comes from 2 38 to a seven |
|
| 195:20 | comes from 2 35 and 208 comes thorne. There's also led to a |
|
| 195:26 | , which When I made this table years ago, uh I got it |
|
| 195:33 | a very picky source. You'll notice it says that 206 is stable, |
|
| 195:38 | 207 has a half life of one 10 to the 17th years. That's |
|
| 195:43 | darn stable. A half life of to the 17th years. How many |
|
| 195:51 | lives have we gone through in the of earth? How many half lives |
|
| 195:55 | two or four have we gone We've gone through 10 to the minus |
|
| 196:04 | 8, 1, 1, 100 of a half life have we gone |
|
| 196:10 | ? That's pretty darn stable. But decided we're not going to call led |
|
| 196:14 | a four stable, we're going to that's a half life of 10 to |
|
| 196:17 | 17th years. Okay, Nobody, corrects for the decay of 204, |
|
| 196:23 | don't think, but it's good that have it, you know, even |
|
| 196:28 | it's a little bit unstable because otherwise wouldn't be able to have what he's |
|
| 196:33 | isotopes. Look at all these other who have half lives of minutes and |
|
| 196:37 | and whatnot. But because we've got isotope here, that's stable and not |
|
| 196:42 | genic. We can do some of normalizing stuff that we were doing |
|
| 196:48 | Um the decay of uranium 2 38 story here is, you know, |
|
| 196:52 | red the red arrows are alpha The blue arrows are beta decay. |
|
| 196:56 | you see some of these things are decays, which means sometimes they go |
|
| 197:00 | way and sometimes they go this It's a percentage is just the flip |
|
| 197:02 | a coin. But the good news no matter which way you go on |
|
| 197:06 | of these branch choices, you always up that led to a six. |
|
| 197:11 | look at the individual case a little closely, uranium 2 38. The |
|
| 197:16 | to thorium 2 34 out of the life of 24 days. All |
|
| 197:19 | Not a big deal. The next hours. Then we get to uranium |
|
| 197:23 | 34. It's got a halfway for years. That can be useful in |
|
| 197:27 | instances. Not one. We're going talk about the dating really young |
|
| 197:32 | It can sometimes be a thing Thorium 30 then has a half life of |
|
| 197:37 | years. That will actually be a in one system. We'll talk about |
|
| 197:42 | . Then the rest of these have short half lives here. Here's 16,000 |
|
| 197:47 | , yeah, 1000 years, minutes seconds, days and minutes and seconds |
|
| 197:54 | then down and it gets to lead a six. So some of these |
|
| 197:58 | , you know, remarkably trivial half . Some are thousands of years. |
|
| 198:05 | the concern about the intermediate chain really away once you give it enough |
|
| 198:10 | Um when the activities of all of isotopes are the same, the system |
|
| 198:16 | in equilibrium. Remember activity is the constant times the abundance. That's the |
|
| 198:24 | number of decays. And so If have a little bit of, if |
|
| 198:29 | have a little bit of the isotope it's decaying a lot. That's the |
|
| 198:32 | as if you have a bunch of decaying slowly and once the activity of |
|
| 198:37 | those little buckets in this chain are same. It's as if you decay |
|
| 198:41 | uranium and go directly to that. can ignore that stuff in the |
|
| 198:44 | But that stuff in the middle takes three or 400,000 years to get all |
|
| 198:48 | . And so before you get to years, the system has got a |
|
| 198:54 | . Although you go back to something the something here, the ratio of |
|
| 199:00 | 2 34 to thorium 2 30 Before gets to be in equilibrium with the |
|
| 199:07 | of those two things being the You can measure its degree of |
|
| 199:11 | that's a measure of age. And for young volcanic rocks that are younger |
|
| 199:15 | , say, 300 million years, can use the degree of dis equilibrium |
|
| 199:21 | date that rock, that's a little complicated. We're not gonna go into |
|
| 199:25 | now, but that's another way to it. But only if you've got |
|
| 199:28 | volcanic rock, it, you less than half a million years |
|
| 199:32 | That's not usually a problem for the industry. So we won't bother with |
|
| 199:36 | . Um the decay of uranium 2 same story. You've got some |
|
| 199:43 | the case in beta decays. Sometimes branch decays. Uh but no matter |
|
| 199:48 | you take it, you always end at the same place, lead |
|
| 199:52 | And look at the individual, the you've got, you know, these |
|
| 199:57 | , these are mostly much shorter half . Here's here's one that's 32,000 |
|
| 200:01 | but the rest are hours and a couple of years, 56 seconds |
|
| 200:08 | of a microsecond. We get to 207 And then story into 32. |
|
| 200:15 | story, only a little less Not so many branch decays, not |
|
| 200:21 | many decays in general. And we uh and and the and the and |
|
| 200:25 | half lives of these intermediate decays are pretty short. Here's just days, |
|
| 200:30 | and years, here's seconds and microseconds we get to lead 208. |
|
| 200:37 | so if we look at the history the parents and daughters on a graph |
|
| 200:42 | this, we can see that the dotted lines of the daughters and the |
|
| 200:46 | lines of the parents and of course daughters are getting bigger and the parents |
|
| 200:49 | getting smaller and the story, values are flat and the 1 to |
|
| 200:57 | values are steep Because the red has shortest half life changing classes changing up |
|
| 201:04 | or down here. Whereas 32, the longest half life. So it's |
|
| 201:09 | slowly and slowly. You can I think Ron's in this system |
|
| 201:19 | And that's why I said you can led to A four as your normalizing |
|
| 201:25 | and it is done sometimes, but a lot really. The only time |
|
| 201:29 | see this done is make, excuse . So you can do, you |
|
| 201:36 | do the lead 207 to uranium 2 or uranium 2 38 can be 2 |
|
| 201:42 | can be used and will be used some carbonates if they have a relatively |
|
| 201:46 | amount of uranium in them. And could do the same thing for 2 |
|
| 201:50 | or the same thing for thorium 2 . But it's not done very much |
|
| 201:57 | we can do another thing that takes of the fact of this pair |
|
| 202:03 | Two isotopes of uranium are decaying to isotopes of lead. So rather than |
|
| 202:08 | look at them one at a time those individual ice crimes, there's ways |
|
| 202:12 | pair them together and make it more discussion. And we can start by |
|
| 202:19 | at the equations for these things You've and in this case we're just going |
|
| 202:25 | call the lead 207 and led to six star, which means radio gen |
|
| 202:31 | . But in this case we are to assume that basically the amount of |
|
| 202:35 | to begin with is close to So I mean radio genic means measured |
|
| 202:41 | initial, but in this case the was probably low. So anyway, |
|
| 202:46 | for for completeness, let's call that radio, the radio genic lead. |
|
| 202:50 | we've got these two equations. And just just to make you clearly understand |
|
| 202:55 | when it says lambda sub eight, means the decay constant for uranium 2 |
|
| 203:01 | is just a shorthand rather than writing 238 and lambda five meets 2 |
|
| 203:07 | And so we've got two equations So if we were to take ages |
|
| 203:13 | plug them into both equations, we numbers out on each side, |
|
| 203:17 | So if we put in a million for one and the other, we |
|
| 203:20 | two values. And so we say samples with uranium lead ages, these |
|
| 203:26 | systems that are equal, are said be concordant. And so what we |
|
| 203:29 | do is plug in a bunch of into both equations and pluck those pairs |
|
| 203:34 | numbers on a diagram. And that be this red line. And the |
|
| 203:39 | on that red line refer to millions years. And so all the points |
|
| 203:45 | that red line have the same age both uranium lead systems and that therefore |
|
| 203:50 | are said to be concordant. And diagram is sometimes called the Concordia |
|
| 203:57 | Now, why isn't that red line ? Why does it curve? Why |
|
| 204:28 | these lines different shapes? This is change of these isotopes over time. |
|
| 204:36 | . They've got different slopes. Why isotopes? They changed the same over |
|
| 204:52 | . What would they have to have have to have the same half |
|
| 204:55 | Right. They're not saying half life different. And so this is just |
|
| 205:02 | that the ratio of one of the changes over time because they're not decaying |
|
| 205:05 | the same rate. So we're using ice stoves but we get curved line |
|
| 205:11 | they're different. Okay, so uh we can also do another thing and |
|
| 205:26 | these two equations and divide one by other and rearrange them. And we |
|
| 205:30 | get this this this equation that says the ratio of lead 207 to lead |
|
| 205:36 | R two. Radio genic lead isotopes going to be a unique function of |
|
| 205:43 | because this ratio here of the two isotopes that's just a constant, |
|
| 205:48 | And then then so are all these numbers except T. So if we |
|
| 205:53 | in T to this equation we will will then predict a 207206 ratio. |
|
| 205:59 | Now unfortunately we can't solve this equation T. Because it's the exponents thing |
|
| 206:06 | . But we can establish a table values. We can just plug in |
|
| 206:09 | bunch of teas and then get this over here. And I've made this |
|
| 206:14 | Very grossly from 1 to 3.2 billion . Just just just so I can |
|
| 206:19 | some changes in the numbers. If rather have it be from from 37 |
|
| 206:24 | to 37.5 million the numbers won't change you can do the same thing. |
|
| 206:29 | so if you measure I rather had in the lab you'll get a ratio |
|
| 206:35 | those two values. You can read this table to say well that suggests |
|
| 206:38 | age of whatever. So going back the Concordia diagram that it is the |
|
| 206:46 | that if everything goes well. The that you analyze the data that you |
|
| 206:52 | will come as a point on this that will sit on the Concordia diagram |
|
| 206:56 | on the red line. But sometimes does and we'll discuss why in a |
|
| 207:01 | . But first of all let's just a point that doesn't fall on that |
|
| 207:05 | . What if it's there and they more often fall below the red line |
|
| 207:09 | above it. So let's just just below the line for a minute. |
|
| 207:20 | We've got three choices now we can at the that we can just go |
|
| 207:25 | from the X. Axis and hit Concordia diagram there And where that |
|
| 207:34 | that corresponds to an age which we the 207-35 age. Or sometimes that |
|
| 207:39 | be abbreviated as the 75 age because likes to say Led to a 70 |
|
| 207:45 | 235 all the time. So that's the 75 age. That's that's that's |
|
| 207:50 | that way. But you could all read across this way to this point |
|
| 207:55 | , that's the 68 H. And see they're very different depending on how |
|
| 207:59 | away this is from Concordia. Those are different. And then the third |
|
| 208:04 | would be to take a point from origin through our point here and where |
|
| 208:10 | hits up here that will correspond to 76 age. Um So which one |
|
| 208:17 | those is best not always clear It depends on a lot of |
|
| 208:23 | What's next? We're gonna talk about . Okay I'm almost out of voice |
|
| 208:30 | that we're almost out of time. So let's go back to think about |
|
| 208:36 | face most commonly dated. We like . It's got a high uranium |
|
| 208:41 | low lead concentration, high closure temperature in all environments. I'm gonna talk |
|
| 208:49 | this in these classes which are you , given in 2022. This some |
|
| 208:57 | these things aren't important anymore. If were in a lab lab we have |
|
| 209:01 | at u of h many labs around world. We now have the |
|
| 209:06 | The measure, just even a little portion of a single zircon, But |
|
| 209:13 | lots of data you might look at literature from say 20 years ago, |
|
| 209:17 | more. when they didn't usually date zircon at a time, they would |
|
| 209:23 | them together because they needed, you , remember I said there are three |
|
| 209:26 | . Three ways to fix our problems sampled the old sample, have a |
|
| 209:30 | of parents or the sample be Well what what is big, depends |
|
| 209:35 | what machine you're using, what what is big today is a lot |
|
| 209:41 | than it used to be. So used to be that when I was |
|
| 209:44 | undergraduate, I was worked in the where I was in charge of taking |
|
| 209:49 | granite and getting some zircons out, we had they brought me two buckets |
|
| 209:54 | of granite, £200 of granite, to get enough zircons. Nowadays you |
|
| 210:00 | a granite this size, you'll find zircons because you only need a couple |
|
| 210:05 | zircons to get a good answer. back then they just had to shovel |
|
| 210:08 | zircons into the mass spectrometer and I , you know what I mean by |
|
| 210:12 | is where nowadays, you know, can just pick three or four beautiful |
|
| 210:17 | and get it done. Um Back , you had, you know, |
|
| 210:22 | zircons weren't enough to provide enough lead measure. You needed 25 50 maybe |
|
| 210:28 | how big they were. So you all 50 of them into this acid |
|
| 210:33 | and dissolve them up and measured all lead and all the uranium and all |
|
| 210:37 | or four dozen zircons. Obviously that disadvantages that you're averaging them together. |
|
| 210:44 | found back in those days that you get us, you could get sort |
|
| 210:51 | micro averages variations in these things. you segregated the zircons first by these |
|
| 210:58 | , by maybe their size by their , their shape or their magnetic |
|
| 211:05 | Now size and magnetic susceptibility are better they are more automated. You can |
|
| 211:10 | a sieve in there and sit the ones from the little ones and the |
|
| 211:14 | susceptibility. There's a machine that's called Friends is a dynamic magnetic separator that |
|
| 211:20 | separate the samples based on their magnetic . More automated. You pour the |
|
| 211:25 | in there and come back a few later and the magnetic ones are over |
|
| 211:28 | and the non magnetic ones are over , color and shape. You have |
|
| 211:33 | sit at the microscope and move the ones over here. The lavender ones |
|
| 211:36 | here and the needle ones over here the stubborn ones over here and that |
|
| 211:41 | forever. Ah But they did all those things to produce different fractions of |
|
| 211:49 | . And when they did that, would get data that sometimes looked like |
|
| 211:53 | and these might be then all the a secular ones and these are the |
|
| 211:58 | stumpy ones or these are the magnetic and the more magnetic ones. Even |
|
| 212:02 | magnetic. But they found that when did this they would get spread on |
|
| 212:07 | thing and this was good and they put it on this diagram. And |
|
| 212:14 | diagram is called the Concordia diagram. also sometimes called the Wetherill diagram is |
|
| 212:18 | guy named George. Wetherall was the one to suggest to be used this |
|
| 212:23 | and why this is important because if get a spread on this then you |
|
| 212:29 | start to interpret this by looking at line and so on. The weather |
|
| 212:34 | diagram. We call that Kirby the Concordia line. And the other |
|
| 212:38 | we call the Discord E. Line. And the Discord E. |
|
| 212:42 | . Will help us interpret these data are not perfect. Um And with |
|
| 212:49 | Discord E. A. You will have uh an intercept with Concordia of |
|
| 212:55 | and another one down low. So are we going to interpret those? |
|
| 213:01 | the upper intercept has usually interpreted either the age of crystallization or the age |
|
| 213:08 | an in heritage component. Which I'll . I'll explain that more in a |
|
| 213:14 | . The lower intercept can be interpreted the age of crystallization or the time |
|
| 213:19 | lead loss. And you may have that there's an interesting thing is that |
|
| 213:23 | age of crystallization can be either one these things. Um but the uh |
|
| 213:29 | way to sort out, which which is usually pretty straightforward if you |
|
| 213:32 | some knowledge of the local geology. So let's imagine then this where we |
|
| 213:46 | a a sample in red there, is say 1700 million years old. |
|
| 213:56 | then for some reason, well, either we have new growth of zircon |
|
| 214:02 | some older cons and this happens quite lot. But you'll have new growth |
|
| 214:06 | it. You have an old, know, inside and a young |
|
| 214:11 | And in in those older studies, could never separate the two. You |
|
| 214:15 | just say that on average this, know, you have a billion year |
|
| 214:19 | zircon and then brand new zircon on outside. And you measure that |
|
| 214:22 | it'll show up as well somewhere between billion and zero depending on the relative |
|
| 214:27 | of the old part and the juvenile . And that might be what's going |
|
| 214:31 | here. We if we grow some cons on these old, this one |
|
| 214:35 | almost entirely new. This one's entirely , but they would have a spread |
|
| 214:41 | a, on a diagram like And we could then interpret that as |
|
| 214:45 | old bed and the younger. Another to consider this is that maybe there |
|
| 214:50 | some sort of lead loss and I'll more lead loss tomorrow morning, but |
|
| 214:55 | could lose, we could lose lead it would mathematically look the same. |
|
| 215:01 | then let's down so that there's an , either a loss event or a |
|
| 215:05 | event and it happens, then we that then nothing happens and we evolved |
|
| 215:11 | over time. Such that this thing was important is still important and moved |
|
| 215:15 | here. This one was here has here and so forth. And now |
|
| 215:19 | have these points here and we can again look at the upper intercept in |
|
| 215:24 | lower intercept and decide how to how interpret them. Um I'm gonna skip |
|
| 215:30 | and I'm going to I'm gonna stop . This is a good place to |
|
| 215:38 | . My voice is getting hard and a lot of complications that I think |
|
| 215:42 | should just begin again tomorrow morning Um so we'll start again here at |
|
| 215:48 | PM. Yeah. So do I to call him or something? Should |
|
| 215:57 | , can I just turn this Yeah. Find him, |
|